Figures & data
Figure 1. Monensin inhibits the proliferation in human colorectal cancer cells. (A) Crystal violet staining assay for RKO cells treated with increasing concentrations in two indicated times. Monensin effectively inhibits the proliferative activity of RKO cell in a dose-dependent and time-dependent manner (a). The optical absorbance for crystal violet staining assay at 48 h post-treatment significantly declines with increasing concentrations of monensin in RKO cell (*, p < 0.05) (b). (B) Crystal violet staining assay for HCT-116 cells treated with increasing concentrations in three indicated times. Monensin effectively inhibits the proliferative activity of HCT-116 cell in a dose-dependent and time-dependent manner (a). The optical absorbance for crystal violet staining assay at 48 h post-treatment significantly declines with increasing concentrations of monensin in HCT-116 cell (*, p < 0.05) (b). Each assay condition was done in triplicate.
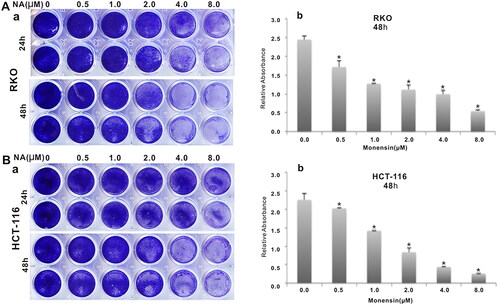
Figure 2. Monensin inhibits the cell migration in human colorectal cancer cells. (a) Cell wounding/migration assay for RKO cells treated with indicated concentration of monensin after the freshly confluent monolayer cells were wounded. (b) Cell wounding/migration assay for HCT-116 cells treated with indicated concentration of monensin after the freshly confluent monolayer cells were wounded. Each assay condition was done in triplicate.
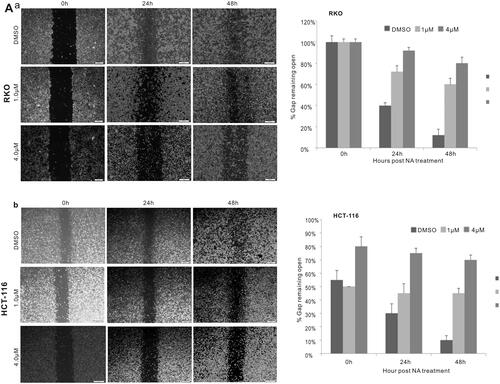
Figure 3. Monensin induces apoptosis in human colorectal cancer cells. (A) Hoechst 33258 staining assay for RKO (a) and HCT-116 (b) treated with varied concertrations of monensin at 24 h post-treatment. Apparent apoptotic cells were counted in at least 10 random felds under 100× magnification. The apoptotic rate rose significantly in monensin-treated RKO and HCT-116 cells (*, p < 0.05) (c). (B) Annexin V-FITC flow cytometry analysis for RKO (a). (b)*, p < 0.05 (monensin-treated group vs. control group). (C) Annexin V-FITC flow cytometry analysis for HCT-116 (a). (b)*, p < 0.05 (monensin-treated group vs. control group). Each assay condition was done in triplicate.
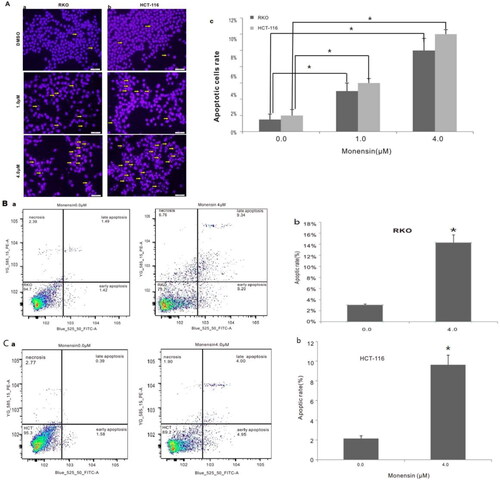
Figure 4. Monensin induces cell cycle progression in human colorectal cancer cells. (A) Cell cycle analysis for RKO in monensin-treated group (a) as well as control group (b). (c)*, p < 0.05 (monensin-treated group vs. control group). (B) Cell cycle analysis for HCT-116 in monensin-treated group (a) as well as control group (b). (c)*, p < 0.05 (monensin-treated group vs. control group). Each assay condition was done in triplicate.
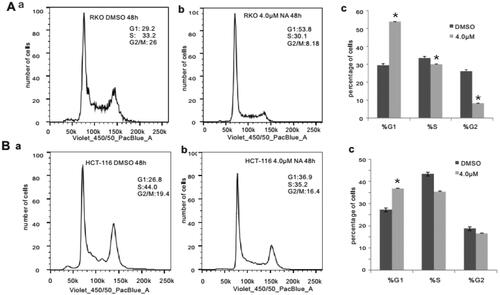
Figure 5. Monensin inhibits multiple cancer-related signaling pathways, including the downstream effectors of IGF signaling. (A) Effect of monensin on the 12 cancer-related pathway reporters pathways. ElK1, Ap1 and Myc/max were significantly attenuated in RKO cells exposed to either 2 µM or 4 µM of monensin at 48 h post-treatment (*, p < 0.05). (B) 4 µM concentration of Monensin effectively inhibited the expression of genes, including K-ras, c-myc, Pi3K and Akt1.But no dose-dependent fashion in monensin-induced inhibition was showed. The augment in K-ras expression, instead of inhibition, was found in group with 2 µM of monensin, while the inhibition to Akt1 was stronger in group with 2 µM of monensin than in group with 4 µM of monensin. An ascending tendency was showed in the expression of IGF1 when the cells treated with increasing concentrations of monensin, whereas a descending tendency was found in the expression of IGF1R. Each assay condition was done in triplicate.
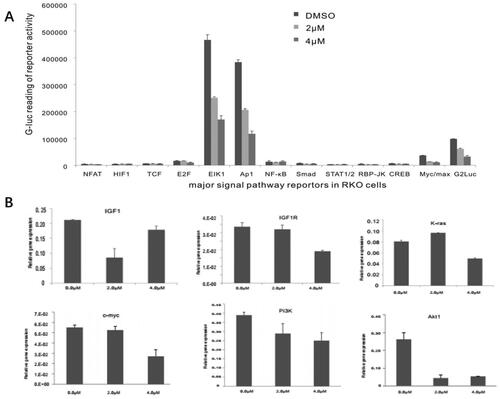
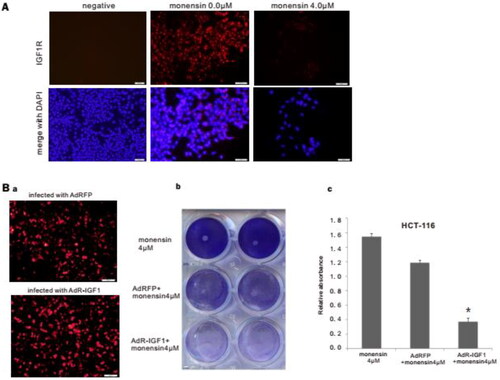
Figure 6. Monensin effectively inhibits the expression of IGF1R, and may suppress the proliferation of human colorectal cancer cells via IGF1R inhibition. (A) Immunofluorescence staining for IGF1R in HCT-116. Monensin effectively inhibited the expression of IGF1R in HCT-116 cells. (B) Immunofluorescence staining assay and crystal violet staining assessment. (a) Immunofluorescence staining for AdRFP and AdR-IGF1. (b) The crystal violet staining at 48 h post-treatment demonstrated that transfection with AdR-IGF1 effectively amplified the inhibitory effect of 4 µM of monensin on cell proliferation in human colorectal cancer cells HCT-116. (c) The optical absorbance for crystal violet staining assay at 48 h post-treatment significantly declines with when the HCT-116 cells were monensin-treated with transfection of AdR-IGF1 (*p < 0.05). Each assay condition was done in triplicate.
Data availability statement
On reasonable request to the corresponding author.
