Figures & data
Figure 1. Experimental design. Groups (n = 8) of 6 week-old female NZBWF1 mice were initiated on either control (CON), low DHA, or high DHA AIN-93G diets. At 8 weeks of age, groups were then intranasally instilled with 1.0 mg cSiO2 or vehicle weekly for 4 weeks. Cohorts were euthanized at 12, 16, 20, or 24 weeks of age, corresponding to 1, 5, 9, or 13 weeks post final instillation (PI). Plasma and BALF were collected for AAb analysis using a high throughput AAg microarray panel for IgG, IgM, and IgA isotypes.
Table 1. Autoantibody classification.
Figure 2. cSiO2-induced IgG AAb responses in BALF are impeded by DHA intake. Heat maps with unsupervised clustering (Euclidian distance method) depict Ab-score values for IgG AAbs for 122 AAgs measured in the BALF. Top bar indicates the VEH/CON, cSiO2/CON, cSiO2/Low DHA, and cSiO2/High DHA groups, respectively, at 1, 5, 9, or 13 weeks PI. Scale bar values reflect the range of variance-stabilized Ab scores, which were centred across rows.
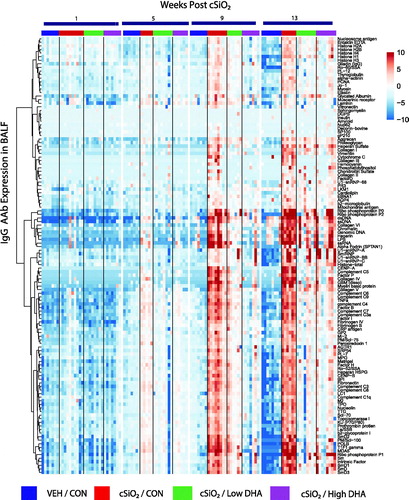
Figure 3. cSiO2-induced IgG AAb responses in plasma are inhibited by DHA intake. Heat maps with unsupervised clustering (Euclidian distance method) depict Ab-score values for IgG AAbs to 122 AAgs measured in the plasma. Top bar indicates the VEH/CON, cSiO2/CON, cSiO2/Low DHA, and cSiO2/High DHA groups, respectively, at 1, 5, 9, or 13 weeks PI. Scale bar values reflect the range of variance-stabilized Ab scores, which were centred across rows.
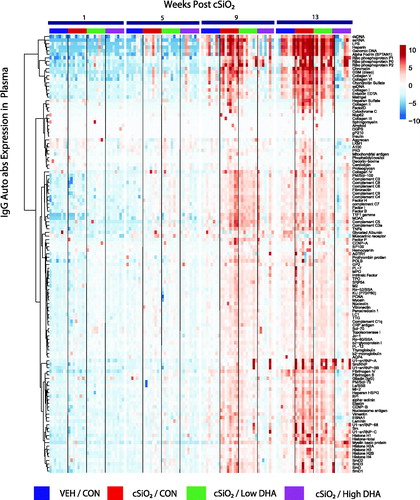
Figure 4. cSiO2-triggered IgG and IgM AAb responses in BALF precede those in plasma in NZBWF1 mice fed control diet. (A) Time course of total IgG and IgM AAb responses in BALF and plasma of mice. AAb expression data were calculated as the summation of 122 Ab-scores (Σ Ab-score) for VEH- and cSiO2-treated mice fed control diet at 1, 5, 9, or 13 weeks PI. Data are mean ± SEM. Asterisk indicates significant difference (p ≤ .05) between cSiO2/CON and VEH/CON mice as calculated by t-test. (B) PCA of differentially expressed IgG or IgM AAb in BALF or plasma of VEH/CON and cSiO2/CON mice. The dashed ellipses show 95% confidence intervals.
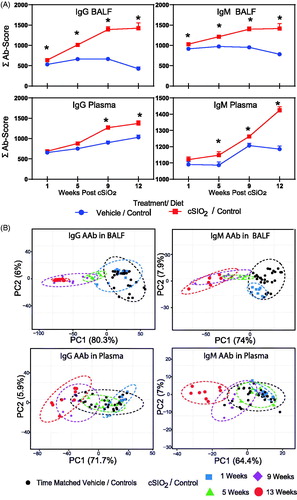
Figure 5. DHA supplementation dose dependently suppresses cSiO2-induced IgG AAb expression in BALF (A) and plasma (B) over time. Ab-score data were obtained using the microarray panel for vehicle (VEH)- or cSiO2-exposed mice fed CON, low DHA, or high DHA diets. Σ Ab-score refers to sum of Ab-scores for AAbs belonging to a particular category (). One-way ANOVA was used to compare experimental groups at selected time points followed by Tukey's HSD method as multiple comparison test. Data are mean ± SEM. Symbols indicate significant difference (p ≤ .05) as follows: * for cSiO2/CON vs. VEH/CON; # for cSiO2/CON vs. cSiO2/Low DHA; and † for cSiO2/CON vs cSiO2/High DHA.
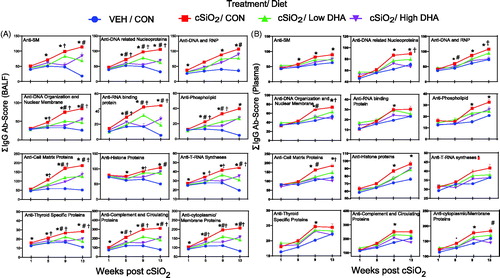
Figure 6. DHA supplementation dose dependently suppresses cSiO2 induction of selected lupus-related IgG AAbs in BALF (A) and plasma (B) over time. Ab-score data were obtained using the microarray panel for vehicle (VEH)- or cSiO2-exposed mice fed CON, DHA low, or DHA high diets. Relative AAb responses for selected AAgs were determined by dividing individual Ab-score by the mean Ab-score for VEH-treated mice fed control diet at 1 week PI. One-way ANOVA was used to compare experimental groups at selected time points followed by Tukey's HSD method as multiple comparison test. Data are mean ± SEM. Symbols indicate significant difference (p ≤ .05) as follows: * for cSiO2/CON vs. VEH/CON; # for cSiO2/CON vs. cSiO2/Low DHA; and † for cSiO2/CON vs cSiO2/High DHA.
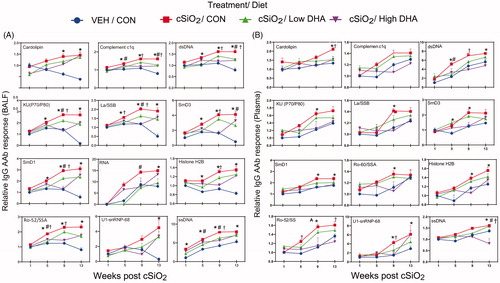
Figure 7. DHA intake inhibits cSiO2-induced expression of IgG AAbs in BALF (A) and plasma (B) specific for AAgs associated other autoimmune diseases. AAbs included those associated with rheumatoid arthritis (collagen II, fibrinogen IV, AAbs fibrinogen S, fibronectin, and vimentin), Sjögren’s syndrome (α-fodrin), systemic sclerosis (topoisomerase I)), vasculitis (MPO and PR3), myositis (Mi-2, TIF1-γ, MDA5), autoimmune hepatitis (LC-1), and coeliac disease (TTG). Relative AAb responses for selected AAgs were determined by dividing individual Ab-score by the mean Ab-score for VEH-treated mice fed control diet at week 1 PI. One-way ANOVA was used to compare experimental groups at selected time points followed by Tukey's HSD method as multiple comparison test. Data are mean ± SEM. Symbols indicate significant difference (p ≤ .05) as follows: * for cSiO2/CON vs. VEH/CON; # for cSiO2/CON vs. cSiO2/Low DHA; and † for cSiO2/CON vs cSiO2/High DHA.
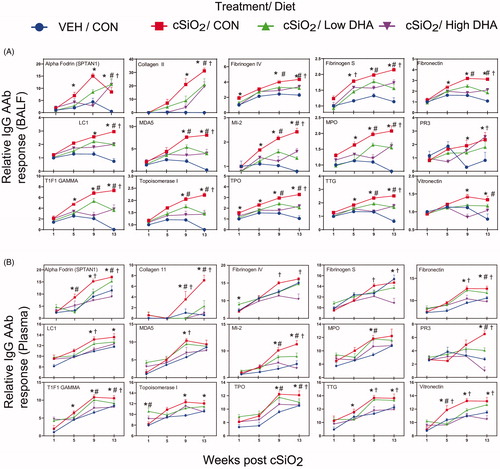
Figure 8. Relationship of IgG AAb responses to ω-3 index in erythrocytes. AAb responses negatively correlate with erythrocytes ω-3 index. (A) For all cSiO2-treated groups, Spearman ρ values were calculated by correlating Σ IgG Ab-score values for specific categories () in BALF and plasma with the ω-3 index in erythrocytes at 1, 5, 9, and 13 weeks PI. Significant correlation values (p ≤ .05) are represented as shaded circles; non-significant correlations are indicated by blank cells. (B) Scatter plots for Σ Ab-score values from selected IgG AAb groups in BALF vs. ω-3 index in erythrocytes at week 9 and week 13. Linear regression lines with 95% confidence intervals (dashed red line) are shown along with the Spearman ρ value and p-value.
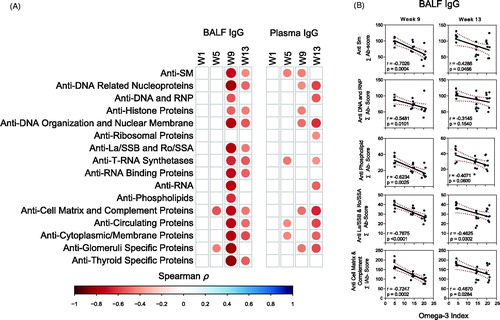
Figure 9. IgG AAb responses in BALF positively correlate with cSiO2-induced gene expression in lung. Scatter plots for Σ Ab-score from selected AAb groups in BALF vs. the Z scores of gene expression in selected immune pathways at week 9. Linear regression lines with 95% confidence intervals (curved lines) are shown along with the Spearman ρ value and p-value.
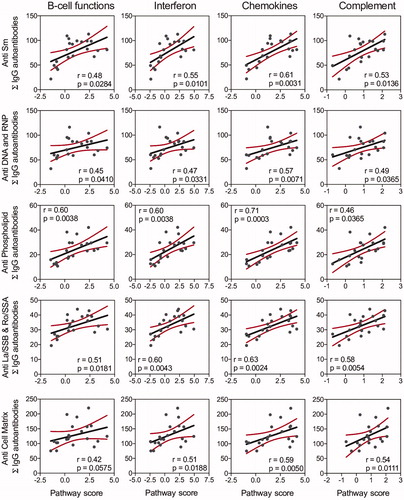
Figure 10. IgG AAb responses in BALF and plasma positively correlate with markers for ELS development. (A) For all cSiO2-treated groups, Spearman ρ values were calculated by correlating Σ IgG Ab-score values with markers for ELS development assessed in lung tissues of mice collected 1, 5, 9, or 13 weeks PI. Σ Ab-score data calculated as the sum of AAbs belonging to a particular category (). Markers for ELS development in lung tissues of mice measured as the percentage of positively stained tissues (CD3, CD45R, and CD21/35) and scores of histopathology (lymphoid aggregates, ectopic lymphoid tissue). Significant correlation values (p ≤ .05) are represented as shaded circles; non-significant correlations are indicated by blank cells. (B) Scatter plots for Σ Ab-score from selected IgG groups in BALF vs. markers for ELS development at 13-week PI. Linear regression lines with 95% confidence intervals (curved lines) are shown along with the Spearman ρ value and p-value.
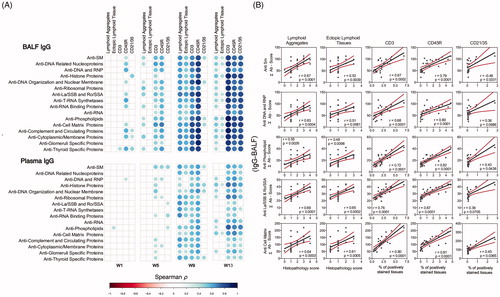
Figure 11. cSiO2-induced IgA AAb responses in BALF at 13 weeks PI are suppressed by DHA supplementation. (A) Heat maps with unsupervised clustering (Euclidian distance method) of 122 AAbs depict Ab-score values for IgA expression in BALF. Top key indicates the VEH/CON, cSiO2/CON, cSiO2/Low DHA, and cSiO2/High DHA experimental groups, respectively. Scale bar values reflect the range of variance-stabilized Ab scores, which were centred across rows. DHA intake suppressed cSiO2-induced IgA AAb responses against (B) selected lupus-related AAgs, and (C) AAgs associated with other autoimmune diseases. Data are Σ Ab-score or specific Ab-scores ± SEM. Different superscript letters on bar graph indicate significant difference (p ≤ .05) compared with the other treatment groups as assessed by one-way ANOVA followed by Tukey's HSD method as a multiple comparison test.
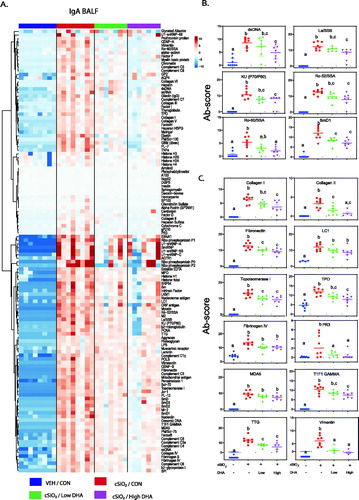
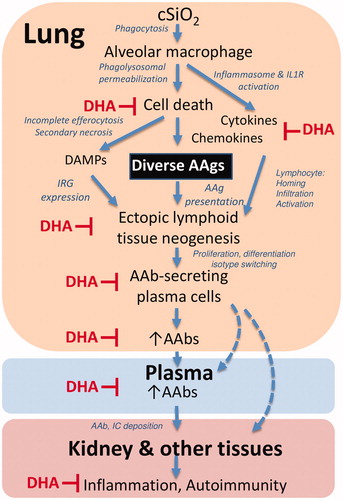
Figure 12. Putative mechanisms for cSiO2 induction of broad autoantibody repertoire by in NZBWF1 mice and targets of DHA suppression. The results presented here and previously indicate that alveolar macrophages phagocytose cSiO2 in the lung, which induces a vicious cycle of phagolysosomal permeabilization, inflammasome activation, proinflammatory cytokine/chemokine release, and cell death. Inability to remove dying cells by efferocytosis results in secondary necrosis, exposure of damage-associated molecular patterns (DAMPs), and a diversity of autoantigens (AAgs). DAMPs stimulate type I interferon-related gene expression (IRG) from plasmacytoid dendritic cells, while cytokines and chemokines recruit and activate additional immune cells. Together, these actions promote the AAg presentation, formation of ectopic lymphoid tissue, and differentiation of B-cells to plasma cells that produce diverse AAbs in the lung fluid and plasma. Upon binding their cognate AAgs, AAbs can form immune complexes (IC) that ultimately deposit in the kidney inflicting damage. The symbol ⊥ indicates steps in this putative pathway that are impaired by dietary DHA supplementation.
Supplemental Material
Download Zip (1.5 MB)SUBMITTED_SUPPLEMENTAL__MATERIALS__LEGENDs__FIGS__TABLE.pdf
Download PDF (6 MB)Data availability statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
