Figures & data
Table 1 Simulated zeolite lattice parameters compared to experiment.
Table 2 Sensitivity on potential parameters of the number of CO2 molecules adsorbed at two test pressures, 5 and 25 bar, to compare with experimental data from Maurin et al. [Citation41].
Table 3 Lennard-Jones potential parameters and partial charges.
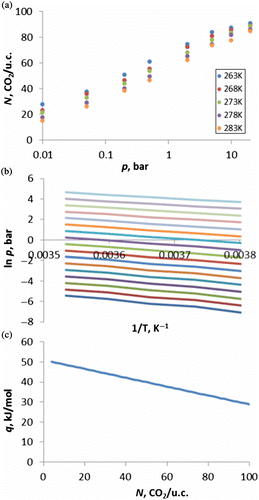
![Figure 11 (Colour online) Adsorption isotherms of CO2 in siliceous MFI, coloured according to temperature. Data points are our simulations, line is experimental data from Yamazaki et al. [Citation54] at 273 K.](/cms/asset/8d54b796-80d5-4cdf-ba07-05421133b34d/gmos_a_839871_f0011_oc.gif)
![Figure 12 (Colour online) Heat of adsorption of CO2 in Na-FAU, Si:Al ratio = 1. Green: test 13, blue: test 14, red: test 15, orange: experimental data from Maurin et al. [Citation41].](/cms/asset/a36b1706-79fc-424b-bdf9-9a1172ddbfef/gmos_a_839871_f0012_b.gif)
![Figure 13 (Colour online) Adsorption isotherm of CO2 in Na-FAU with Si:Al ratio = 1 (green) and Si:Al ratio = 1.18 (red and blue) at 303.2 K. Data points are our simulations, blue line is represents experimental data from Pillai et al. [Citation55].](/cms/asset/651c947c-fc43-4d8a-a8cb-7c442ae4c172/gmos_a_839871_f0013_oc.gif)