Figures & data
Figure 1. (Colour online) Schematic diagram of fixed-bed adsorber. A peristaltic compressor/pump is used to maintain a constant flow rate. In most gas-phase adsorbers, the gas enters from the top and flows down through the bed, while in a typical liquid system the column fills upwards [Citation18]. The outlet of the bed is connected to a sample collector.
![Figure 1. (Colour online) Schematic diagram of fixed-bed adsorber. A peristaltic compressor/pump is used to maintain a constant flow rate. In most gas-phase adsorbers, the gas enters from the top and flows down through the bed, while in a typical liquid system the column fills upwards [Citation18]. The outlet of the bed is connected to a sample collector.](/cms/asset/b5638634-981b-49e1-90c4-2966c30355be/gmos_a_2202757_f0001_ob.jpg)
Table 1. Isotherm models, Henry and saturation regime, and the order of input of the arguments in RUPTURA.
Table 2. Isotherm models and their inverse.
Table 3. Reduced grand potential for isotherm models.
Table 4. Sorption pressures for isotherm models.
Figure 2. (Colour online) IAST algorithm: (a) adsorbed phase mole fraction as a function of the reduced grand potential (b) graphical representation of the basic IAST relationship. The sum of the adsorbed phase mole fractions is unity at reduced grand potential mol/(kg framework). By using
, a root-finding algorithm can be used. Note that that
has a monotonic relation to the reduced grand potential, hence it is also amendable to bi-section methods.
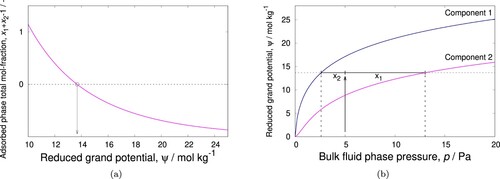
Figure 3. (Colour online) Example of IAST prediction for a binary mixture described by Langmuir-Freundlich isotherms. At 5 Pa total pressure and gas-phase mole fractions and
, we find adsorbed absolute loadings of
and
mol/kg. Typically in a mixture, a component with the highest saturation loading will drive the other components out at high pressures.
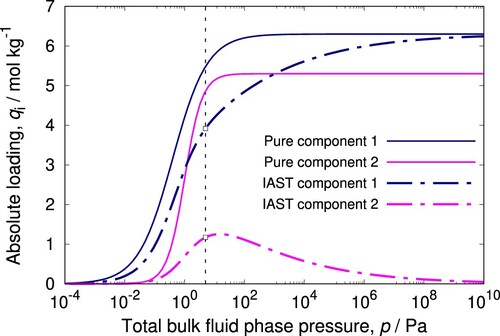
Figure 4. (Colour online) Schematic representation of the Segregated Explicit Isotherm (SEI) model which accounts for the separate thermodynamic equilibrium of the adsorbed phases with the gas phase at different adsorption sites. Each adsorbed phase is separately in thermodynamic equilibrium with the gas phase and it is represented by the adsorption isotherm proposed by Van Assche et al. [Citation30]. The gas phase has a total pressure of and the gas phase mole fractions of the components in the mixture are represented by y. In the adsorbed phase j, the loading of the component i is
.
![Figure 4. (Colour online) Schematic representation of the Segregated Explicit Isotherm (SEI) model which accounts for the separate thermodynamic equilibrium of the adsorbed phases with the gas phase at different adsorption sites. Each adsorbed phase is separately in thermodynamic equilibrium with the gas phase and it is represented by the adsorption isotherm proposed by Van Assche et al. [Citation30]. The gas phase has a total pressure of pT and the gas phase mole fractions of the components in the mixture are represented by y. In the adsorbed phase j, the loading of the component i is qi,j.](/cms/asset/2132e566-8680-4dc6-b3e0-f11ff02fb40c/gmos_a_2202757_f0004_oc.jpg)
Algorithm 1 Procedure for calculating the equilibrium loadings for components in mixtures using the analytic approach of Van Assche et al. [Citation30].
Figure 5. (Colour online) Equilibrium of each of the adsorbed phases with the gas phase in the Segregated Ideal Adsorbed Solution Theory (SIAST) model [Citation29]. Each adsorbed phase is separately in equilibrium with the gas phase. The system is at a constant temperature. The gas phase has a total pressure of and the mole fraction of component i equals
. In the adsorbed phase j, the loading of component i is
.
![Figure 5. (Colour online) Equilibrium of each of the adsorbed phases with the gas phase in the Segregated Ideal Adsorbed Solution Theory (SIAST) model [Citation29]. Each adsorbed phase is separately in equilibrium with the gas phase. The system is at a constant temperature. The gas phase has a total pressure of ptot and the mole fraction of component i equals yi. In the adsorbed phase j, the loading of component i is qij.](/cms/asset/4978ba5b-fe52-4fad-b6c5-4cd64af36645/gmos_a_2202757_f0005_oc.jpg)
Figure 6. (Colour online) Numerical procedure to calculate equilibrium loadings for a binary mixture developed by Mirzaei et al. [Citation118]. The bulk phase concentrations are the input to this approach and the adsorbed mole fraction for component 1 is used as the guessed value. Based on
,
is estimated. Further, the grand potentials for components 1 and 2 are calculated separately and the difference between these two values are computed. If the difference is less than the predefined error value, then the total equilibrium loadings and the loadings for each component are determined. Otherwise, a new guessed value for component 1 is considered and the steps (3–7) are repeated.
![Figure 6. (Colour online) Numerical procedure to calculate equilibrium loadings for a binary mixture developed by Mirzaei et al. [Citation118]. The bulk phase concentrations are the input to this approach and the adsorbed mole fraction for component 1 (x1) is used as the guessed value. Based on x1, x2 is estimated. Further, the grand potentials for components 1 and 2 are calculated separately and the difference between these two values are computed. If the difference is less than the predefined error value, then the total equilibrium loadings and the loadings for each component are determined. Otherwise, a new guessed value for component 1 is considered and the steps (3–7) are repeated.](/cms/asset/2854755e-cd91-4669-980b-851ad61b628f/gmos_a_2202757_f0006_ob.jpg)
Algorithm 2 IAST calculation using bi-section. The tolerance value ϵ can be set to .
Algorithm 3 Inverse computation using bi-section. The tolerance value ϵ can be set to 10–15.
Table 5. Parameters for the O'Brien and Myers adsorption isotherm model considered by Moon and Tien [Citation110] for a 10-component mixture at 300 kPa, mol/kg and
.
Table 6. Parameters for the O'Brien and Myers adsorption isotherm model considered by Landau et al. [Citation74] for a 10-component mixture, mol/kg.
Figure 7. (Colour online) Schematic representation of a packed bed adsorber. The packed bed adsorber is a cylindrical column filled with nanoporous adsorbent material (crystalline or amorphous). When a fluid mixture is fed as input to the column, adsorption of various components present in the mixture takes place inside these nanoporous materials.
Figure 8. (Colour online) Different zones in a breakthrough curve at the exit of the fixed bed column. Fluid phase concentration of an adsorbing component as a function of time at the fixed bed column outlet. The profile is divided into three zones: (a) the unsaturated zone: where the maximum adsorption takes place, (b) mass transfer zone: where adsorption process progresses rapidly towards equilibrium, (c) saturated zone: where no more adsorption takes place as equilibrium between the fluid and the adsorbed phase is achieved.
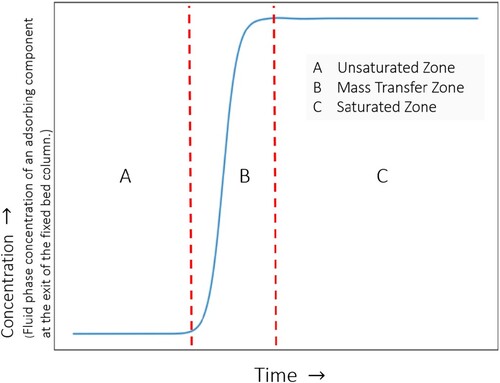
Table 7. Correlations for estimation of axial dispersion coefficient, in fixed bed adsorbers.
Figure 9. (Colour online) Loading and concentration profiles according to the pore diffusion model with external mass transfer resistance. The external mass transfer resistance is modeled using the film flux , which is the linear difference of the concentration
in the bulk solution, and the concentration
at the surface of the crystallite. The flux
is the sum of the pore diffusion and surface diffusion:
. The continuity of the materials fluxes requires
. The surface is considered to be in equilibrium and the adsorbent loading
is calculated from the isotherm with functional form q, where the pressure p is related to the surface concentration
via the ideal gas law as
.
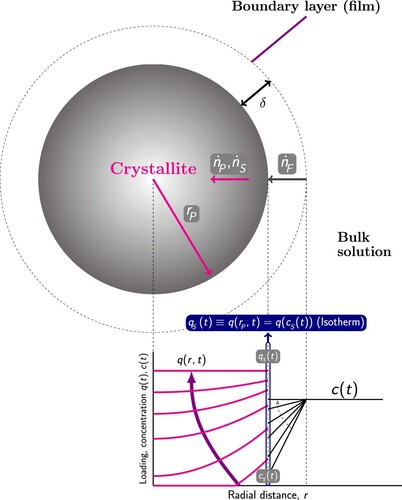
Figure 10. (Colour online) Loading and concentration profiles according to the Linear Driving Force (LDF) model with external mass transfer resistance. The LDF model assumes that there is linear gradient within the solution-side film and within a comparable fictive solid film. The film flux is the linear difference of the concentration
in the bulk solution, and the concentration
at the surface of the crystallite. The solid flux
is the linear difference between the equilibrium loading
at the surface and the average loading
:
, where
is the particle density,
is the intra-particle mass transfer coefficient. The continuity of the materials fluxes requires
. The surface is considered to be in equilibrium and the adsorbent loading
is calculated from the isotherm with functional form q, where the pressure p is related to the surface concentration
via the ideal gas law as
.
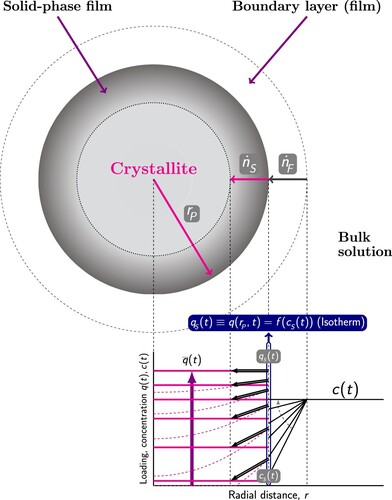
Table 8. First and second order derivatives disretised using the Finite Difference Method (FDM) method [Citation216].
Figure 11. (Colour online) Algorithm to calculate the breakthrough curve. It is based on the method of lines (MOL) technique to solve the system of differential algebraic equations. Time integration is done using an explicit method called Strong Stability preserving Runge-Kutta (SSP-RK(3,3)) as shown in Equations (Equation292(292)
(292) )–(Equation294
(294)
(294) ). We first calculate the temporal derivatives of the partial pressures
and the adsorbed loadings
. Until the final time step is reached, we update the values of
,
,
,
and v at each time step.
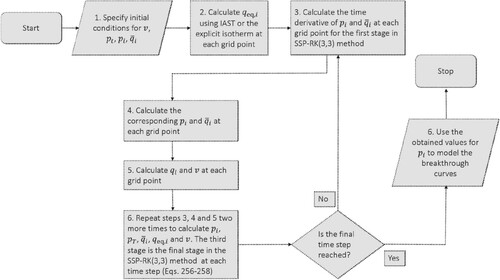
Figure 12. (Colour online) Examples of pulse-style breakthrough curve simulation for the mixture of isomers: n-hexane
, 3-methyl pentane
, 2,2-dimethyl butane
, and 2,3-dimethyl butane
in BEA-type zeolite at 523 K and 12 bar. Isothermal and isobaric conditions are imposed on the fixed bed column of length (a) 0.1 m and (b) 0.5 m. The bed porosity
is 0.2. Helium is used as the carrier gas. The inlet mole fractions for the
isomers are considered to be 0.01975. The interstitial velocity (v) at the inlet is 0.019 ms
. 5s is the pulse time
.
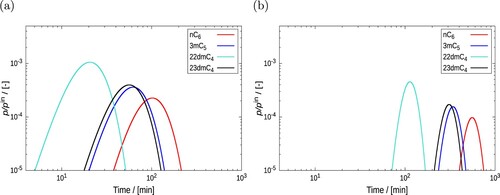
Table 9. Experimental conditions for separation of ,
.
Table 10. Values of dispersion coefficient and mass transfer coefficient (k) for
and
.
Table 11. Pure component adsorption isotherm parameters.
Figure 13. (Colour online) Validation of the breakthrough curve model with the experimental results of Jolimaitre et al. [Citation233]. This figure shows the separation of and
in MFI-type zeolite at 473K and 5bar. The coloured squares represent the experimental data and the solid lines represent the results from our simulations.
![Figure 13. (Colour online) Validation of the breakthrough curve model with the experimental results of Jolimaitre et al. [Citation233]. This figure shows the separation of 2mC4 and 2mC5 in MFI-type zeolite at 473K and 5bar. The coloured squares represent the experimental data and the solid lines represent the results from our simulations.](/cms/asset/b46f38df-d0d9-4b56-881b-fdb3c6d682e6/gmos_a_2202757_f0013_oc.jpg)
Figure 14. (Colour online) Adsorption isotherms for CO and C
H
in MOR-type zeolite at 300 K. (a) Unary isotherms (dual-site Langmuir) which are fitted to the pure component loading data obtained using RASPA, (b) comparison between mixture isotherms for equimolar composition of CO
and C
H
computed using IAST, SIAST and SEI.
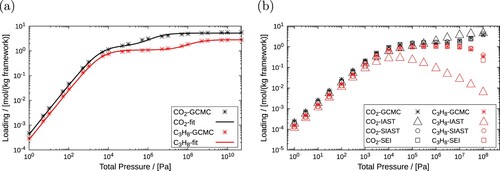
Table 12. Fitted parameters for the adsorption of pure and
in MOR-type zeolite at 300 K.
Table 13. Fitted parameters for the adsorption isotherm (dual-site Langmuir-Freundlich) of o-xylene in MAF-x8-P1 zeolite at 433 K using different methods.
Figure 15. (Colour online) Comparison between breakthrough curves obtained on implementing IAST, SIAST, and SEI to the breakthrough curve model. A mixture of CO and C
H
in MOR-type zeolite at 300 K and 10
Pa is considered. Each of these components constitutes 10% of the gas phase. Helium is used as the carrier gas. The adsorption column is operated in isothermal and isobaric conditions.
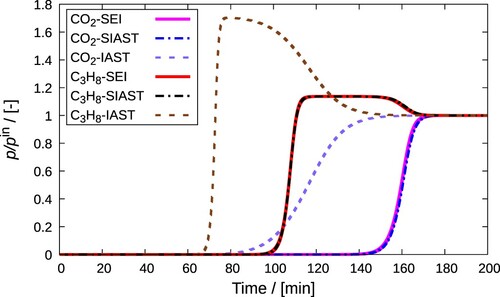
Figure 16. (Colour online) Grid independence test on simulation of breakthrough curves for a mixture of CO and C
H
in MOR-type zeolite at 300 K and 10
Pa is performed. (a) Breakthrough curves simulated at different spatial grid points (
), (b) certain zoomed in parts of the curves showing discrepancies due to the use of a different number of grid points. Each of these components constitutes 10% of the gas phase. Helium is used as the carrier gas. The adsorption column is operated in isothermal and isobaric conditions. With increasing the number of grid points, the breakthrough curves slightly changes. The variation becomes smaller between
.
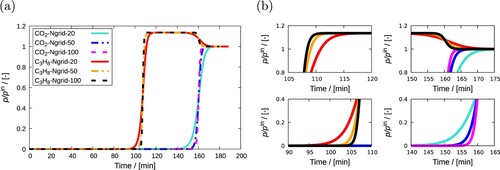
Figure 17. (Colour online) Schematic representation of GA operators crossover, and mutation. The crossover operator is analogous to the crossover that happens during sexual reproduction in biology, and it combines the genotype of two parents to generate new offspring. We implemented three types of crossover operator: with one- and two points, and the uniform crossover. The mutation operator is activated before the children are added to the next generation.

Figure 18. (Colour online) Flowchart of one step, k, in the Nelder-Mead algorithm [Citation117] for a parametric function, f, with two parameters. In this way, the simplex is a triangle, and the visualisation is easier. The four operations and the sorting stage are in green labels.
![Figure 18. (Colour online) Flowchart of one step, k, in the Nelder-Mead algorithm [Citation117] for a parametric function, f, with two parameters. In this way, the simplex is a triangle, and the visualisation is easier. The four operations and the sorting stage are in green labels.](/cms/asset/b7338583-92e8-4046-a061-c2301961b951/gmos_a_2202757_f0018_oc.jpg)
Figure 19. (Colour online) (Left) Adsorption of n-C in BEA zeolite at 552 K and some fitted models using ISOFIT, Gnuplot, IAST++, and RUPTURA for a Toth model of a single adsorption site. (Right) Predicted loading vale vs. observed loading values. The goodness-of-fit in colours (same code colour of the Left panel) for each code. The RMSE values are in [mol/(kg framework)] units.
![Figure 19. (Colour online) (Left) Adsorption of n-C7 in BEA zeolite at 552 K and some fitted models using ISOFIT, Gnuplot, IAST++, and RUPTURA for a Toth model of a single adsorption site. (Right) Predicted loading vale vs. observed loading values. The goodness-of-fit in colours (same code colour of the Left panel) for each code. The RMSE values are in [mol/(kg framework)] units.](/cms/asset/42928ae0-b2f0-4d7f-8307-025306ac2a5c/gmos_a_2202757_f0019_oc.jpg)
Figure 20. (Colour online) (Left) Adsorption of o-xylene in MAF-X8 zeolite at 300 K and some fitted models using IAST++, Gnuplot (from scratch), GAIAST, and RUPTURA for a dual-site Langmuir-Freundlich model. (Right) Predicted loading vale vs. observed loading values. The goodness-of-fit for each code in colours defined in the left panel. The RMSE values are in [mol/(kg framework)] units.
![Figure 20. (Colour online) (Left) Adsorption of o-xylene in MAF-X8 zeolite at 300 K and some fitted models using IAST++, Gnuplot (from scratch), GAIAST, and RUPTURA for a dual-site Langmuir-Freundlich model. (Right) Predicted loading vale vs. observed loading values. The goodness-of-fit for each code in colours defined in the left panel. The RMSE values are in [mol/(kg framework)] units.](/cms/asset/94dfaa78-6a32-4a9f-87a1-5fc370fd32d6/gmos_a_2202757_f0020_oc.jpg)
Figure 21. (Colour online) The adsorption isotherm prediction for two, three, and four sites Langmuir-Freundlich isotherm models for o-xylene in MAF-X8. Without the experimental error information for the loading data is difficult to establish what kind of model is more realistic.
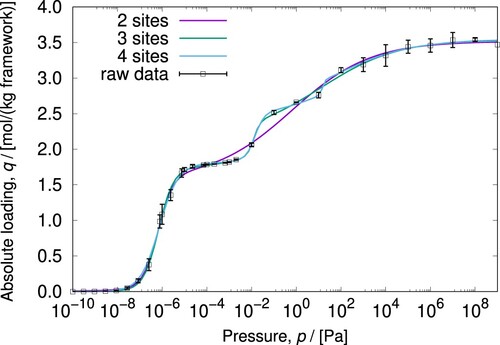
Figure 22. (Colour online) The C-alkane-isomers adsorption isotherms in MAF-6 computed from grand-canonical Monte Carlo simulations fitted with dual- and triple site Langmuir-Freundlich isotherm models. The peculiar shape of these isotherms in the low-pressure region makes this system challenging to preform reliable fitting.
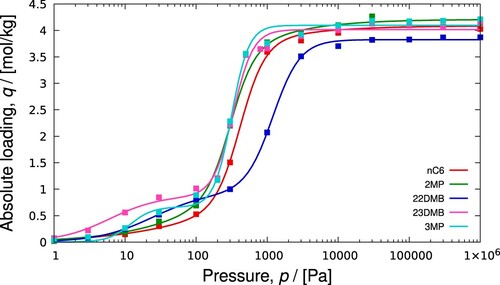
Figure 23. (Colour online) Breakthrough predictions of an equimolar mixture of CO-N
in silicalite at 313 K and
Pa: (a) breakthrough curve plot (file breakthrough_dimensionless.pdf) (b) movie frame of the loading inside the column (file column_movie_Q.mp4).
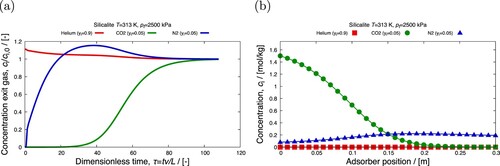
Figure 24. (Colour online) Mixture predictions of a isomer mixture in BEA- type zeolite at 552K using explicit isotherm model developed by Van Assche et al [Citation30]: (a) pure components (file
), (b) mixture prediction (file
).
![Figure 24. (Colour online) Mixture predictions of a C6 isomer mixture in BEA- type zeolite at 552K using explicit isotherm model developed by Van Assche et al [Citation30]: (a) pure components (file pure_component_isotherms.pdf), (b) mixture prediction (file mixture_prediction.pdf).](/cms/asset/30d32a64-e75f-47ed-9f5d-de6c5cb671fa/gmos_a_2202757_f0024_oc.jpg)
Figure 25. (Colour online) Fitting a dual site Langmuir isotherm model to the pure component isotherm data for the adsorption of p-xylene in MAF-X8- type zeolite at 433 K. The pure component adsorbed loadings are computed using grand-canonical Monte Carlo simulations. The fitting is performed using genetic algorithm optimisation which is shown in the file .
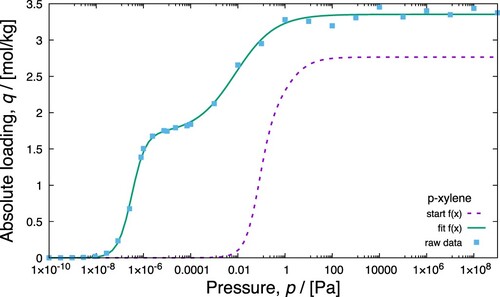
Table 14. List of mixture prediction, breakthrough, and fitting examples provided with RUPTURA.
