Figures & data
Fig. 1. Structures of the okaramine family. Notes: Okaramines A (1), B (2), C (3), D (4), E (5), F (6), G (7), H (8), I (9), J (10), K (11), L (12), M (13a: original; 13b: revised), N (14), O (15), P (16), Q (17), and R (18).
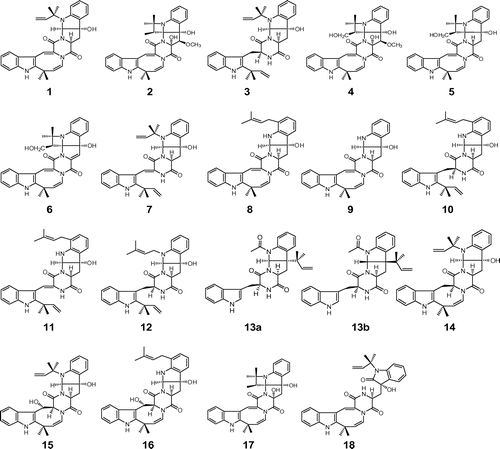
Fig. 2. Structures of bioactive fungal metabolites. Notes: Insecticidal compounds: communesins A (22), B (23), D (19), E (20), and F (21); chrodrimanins A (25), B (26), C (24), D (27), E (28), F (29), G (30), and H (31); convulsive compounds: verruculogen (32); penitrem A (33); 6-bromopenitrem E (34); brasiliamides A (35), B (36), C (37), D (38), and E (39); acetoxydehydroaustin (40), austin (41), dehydroaustin (42), austinol (43), dehydroaustinol (44), and neoaustin (45); paralytic compounds: asperparalines A (46), B (47), and C (48); PF1171 A (49a), epi-PF1171A (49b), PF1171B (50a), epi-PF1171B (50b), PF1171F (51), and PF1171 G (52); cyclopiamines A (53), B (54), C (55), and D (56).
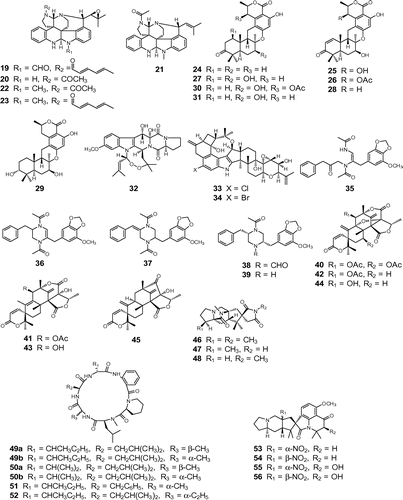
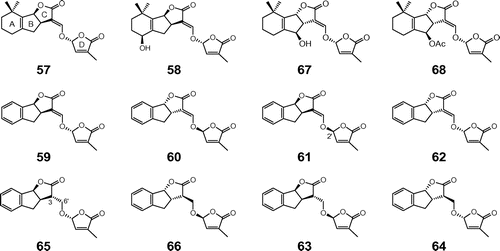
Fig. 3. Structures of natural and synthetic strigolactones. Notes: Natural strigolactones: 5-deoxystrigol (57), strigol (58), orobanchol (67), and orobanchyl acetate(68); synthetic strigolactones: (+)-GR24 (59), (−)-ent-GR24 (60), (+)-2′-epi-GR24 (61), (−)-ent-2′-epi-GR24 (62), (−)-3,6′-dihydro-GR24 (65), (+)-ent-3,6′-dihydro-GR24 (66), (−)-2′-epi-3,6′-dihydro-GR24 (63), and (+)-ent-2′-epi-3,6′-dihydro-GR24 (64).