Figures & data
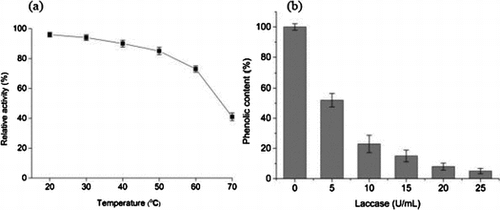
Fig. 1. The time course of laccase secretion by Abortiporus biennis J2 in basal laccase production medium at 30 °C on a rotary shaker at 150 rpm.
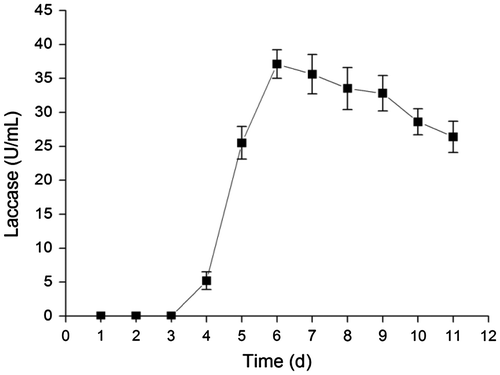
Fig. 2. Effect of different inducers on laccase production of Abortiporus biennis. Cu2+ 0.1 mM, ethanol 0.5 M, tannic acid, vanillin, guaiacol, ferulic acid, and catechol 0.1 mM, respectively.
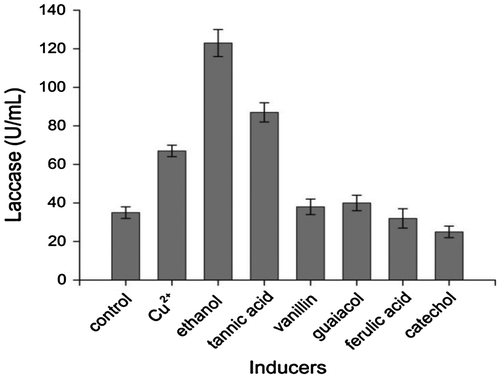
Table 1. Purification of Laccase from Abortiporus biennis J2.
Fig. 3. SDS-PAGE of purified laccase from Abortiporus biennis J2. 1 Standard molecular weight markers, 2 Purified laccase.
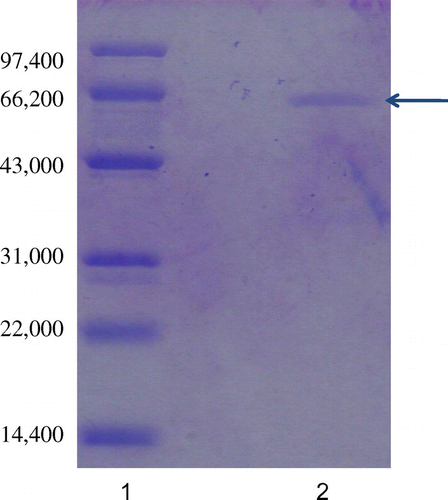
Table 2. Comparison of the N-terminal amino acid sequences of Abortiporus biennis laccase with other fungal laccases.
Fig. 4. Effect of temperature on laccase activity (a) and stability of purified laccase (b) Relative activity on laccase activity was defined as the percentage of activity measured with respect to the maximum enzyme activity. Original enzyme activity on stability of laccase was defined as 100%.
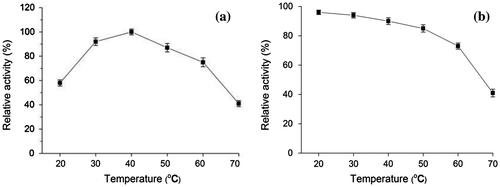
Fig. 5. Effect of pH on laccase activity (a) and stability of purified laccase (b) Relative activity on laccase activity was defined as the percentage of activity measured with respect to the maximum enzyme activity. Original activity on stability of laccase was defined as 100%.
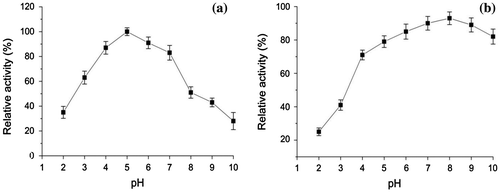
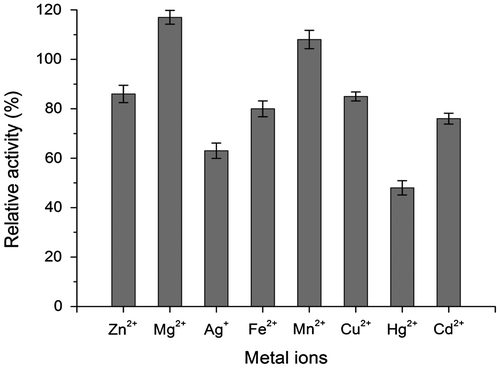
Fig. 6. Effects of heavy metal ion on the laccase activity. The enzyme activity assayed in the absence of heavy metal ions was recorded as 100%.