Figures & data
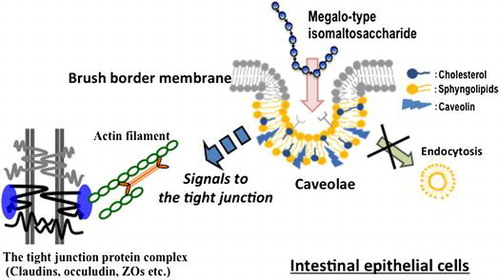
Figure 1. Effects of oligo-type isomaltosaccharide (IMO) or megalo-type isomaltosaccharide (IMM) on the transport of Lucifer yellow (a) and FD-20S (b), which are permeable markers of tight junctions, in a closed loop of the anesthetized rat jejunum for 20 min. The final concentration of the test sugars was 4%(w/v) in the injected fluid. Values are shown as percentages for the amounts of the injected markers and shown as the mean ± SEM (n = 7–8). Means not sharing a common alphabetical letter differ significantly (p < 0.05).
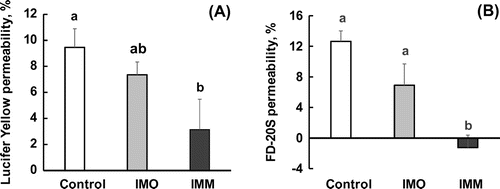
Figure 2. Dose-dependent suppression of the transport of permeable markers of tight junctions, Lucifer yellow and FD-20S, by megalo-type isomaltosaccharide (IMM) in a closed loop of the anesthetized rat jejunum for 20 min. Permeability (%) are values with the control group as 100% and shown as the mean ± SEM (n = 7–8). Means not sharing a common alphabetical letter differ significantly (p < 0.05).
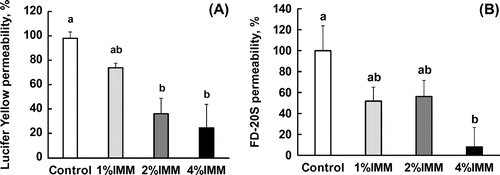
Figure 3. Effects of 2%(w/v) or 4%(w/v) megalo-type isomaltosaccharide (IMM) on transepithelial electrical resistance and the transport rate of the tight junction marker Lucifer yellow (100 μmol/L in apical medium) for 40 min in Caco-2 cell monolayers. Asterisks indicate significant differences compared to control values at each time point (n = 5–6, p< 0.05).
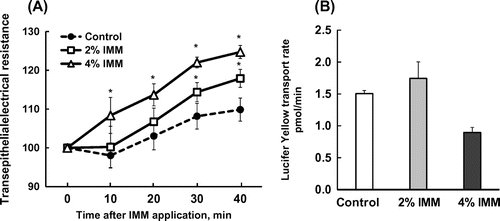
Figure 4. Changes in transepithelial electrical resistance (TEER, A) and the transport rate of Lucifer yellow (B) after application of 4%(w/v) oligo-type isomaltosaccharide (IMO) or 4%(w/v) megalo-type isomaltosaccharide (IMM) and the effects of the sequestration of cholesterol from Caco-2 cell monolayers by pre-treatment with 0.6%(w/v) methyl-β-cyclodextrin (MBC) for 40 min. Asterisks indicate significant differences compared to control values at each time point (n = 5–6, p< 0.05).
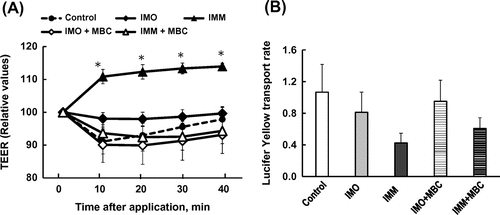
Figure 5. Inhibition of the increase in transepithelial electrical resistance (TEER) after application of megalo-type isomaltosaccharide (IMM) by treatment of Caco-2 cell monolayers with caveolin-1 antibody (1/250 dilution). Asterisks indicate significant differences compared to control values at each time point (n = 5–6, p < 0.05).
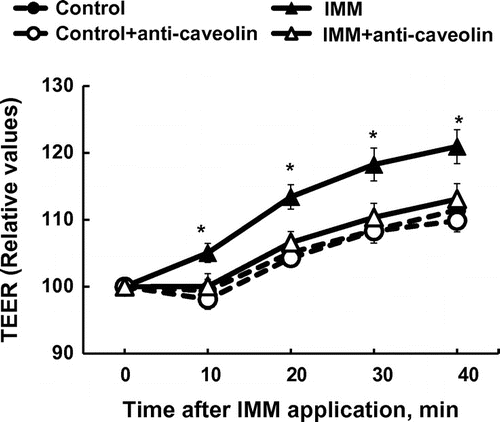
Figure 6. Effects of three endocytosis inhibitors on the increase in transepithelial electrical resistance (TEER) induced by 4%(w/v) megalo-type isomaltosaccharide (IMM) in Caco-2 cell monolayers. Nystatin (50 μmol/L) as a caveolae inhibitor, chlorpromazine hydrochloride (30 μmol/L) as a clathrin-mediated endocytosis inhibitor, and 5-(N-ethyl-N-isopropyi)-amiloride (20 μmol/L) as a macropinocytosis inhibitor were used. Asterisks indicate significant differences compared to control values at each time point (n = 5–6, p < 0.05).
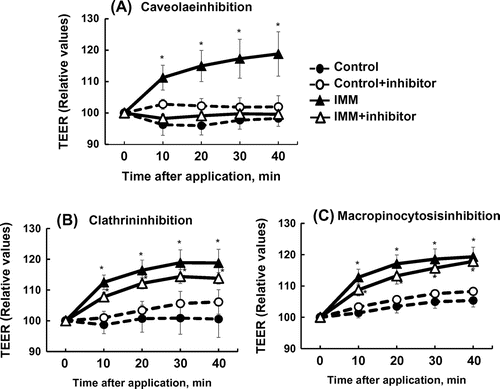
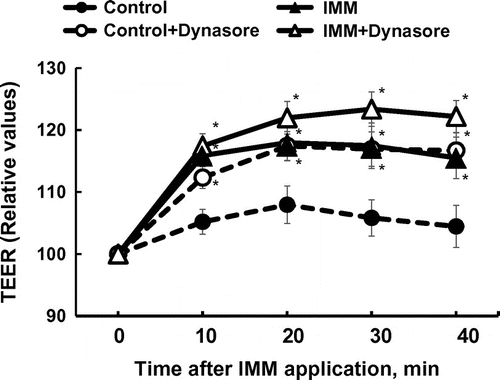
Figure 7. Effects of dynasore (80 μmol/L), an inhibitor of dynamin, an essential component for budding processes in both caveolae- and clathrin-dependent endocytosis, on the increase in transepithelial electrical resistance (TEER) induced by the application of megalo-type isomaltosaccharide (IMM) in Caco-2 cell monolayers. Asterisks indicate significant differences compared to control values at each time point (n = 5–6, p < 0.05).