Figures & data
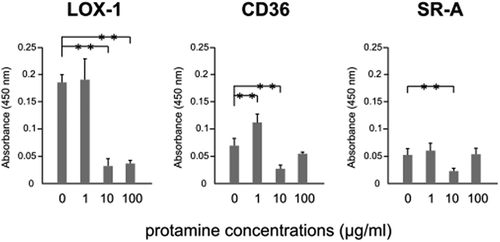
Figure 1. NaCl inhibits the binding of ox-LDL to LOX-1.
Ox-LDL was incubated along with NaCl at the indicated concentration in a recombinant LOX-1-immobilized ELISA system. A colorimetric assay was performed and values are expressed as mean absorbance at 450 nm. Error bars represent the SD for five samples. **P < 0.01.
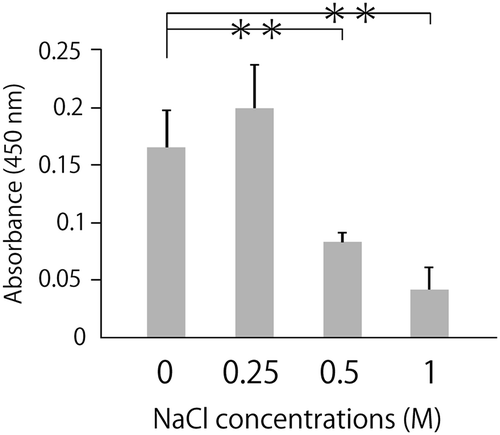
Figure 2. Charged amino acids inhibit the binding of ox-LDL to LOX-1.
Ox-LDL was incubated with amino acids and analyzed using a recombinant LOX-1-immobilized ELISA system. A colorimetric assay was performed and values are expressed as mean absorbance at 450 nm. The X axis shows amino acid concentrations. Error bars represent the SD for five samples. **P < 0.01, *P < 0.05.
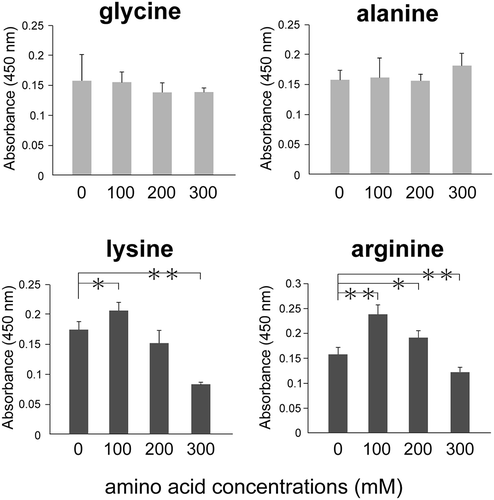
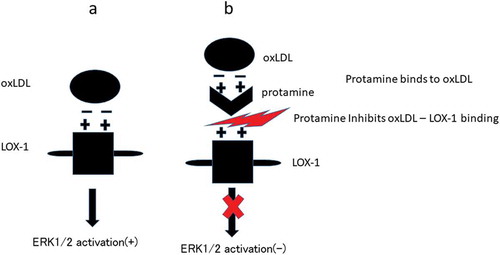
Figure 3. Protamine inhibits the binding of ox-LDL to the receptor.
Ox-LDL was incubated with protamine at the indicated concentration in a recombinant receptor protein-immobilized ELISA system. A colorimetric assay was performed and values are expressed as mean absorbance at 450 nm. Error bars represent the SD for five samples. **P < 0.01.
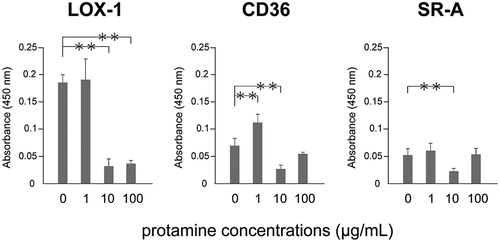
Figure 4. SPR analysis of the interaction of ox-LDL with immobilized protamine.
Sensorgram for ox-LDL with protamine immobilized on a sensor chip. Arrows indicate injection points of sequentially diluted ox-LDL (0.64, 3.2, 16, 80, 400 nM). Dashed line indicates data fitting result.
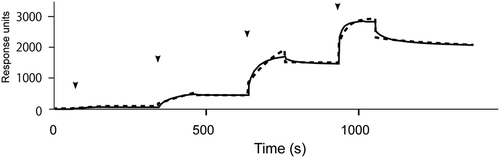
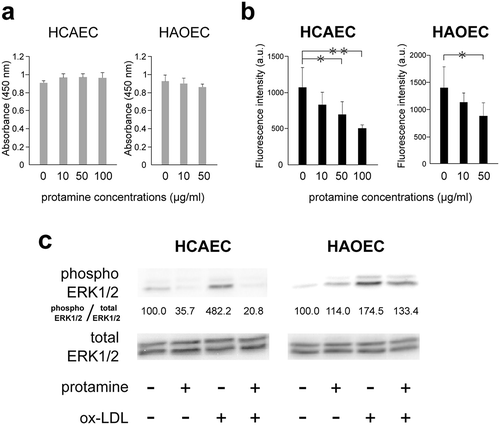
Figure 5. Protamine inhibits the uptake of ox-LDL to endothelial cells.
(a) Effect of protamine on the cell viability of HCAECs and HAOECs. HCAECs and HAOECs were treated with protamine at the indicated concentration for 24h. A cell counting kit was used and values are expressed as absorbance at 450 nm. Error bars represent the SD for eight samples. (b) Protamine inhibits the uptake of ox-LDL in HCAECs and HAOECs. DiI-ox-LDL uptake in HCAECs or HAOECs was measured in the presence of various concentrations of protamine. Values are expressed as fluorescence intensity in arbitrary units. Error bars represent the SD for six samples. **P < 0.01, *P < 0.05. (c) Inhibitory effect of protamine on the activation of ERK1/2 induced by ox-LDL in HCAECs or HAOECs. Endothelial cells were preincubated with protamine and were treated with or without ox-LDL for 10 min. The expression of protein levels of phosphor ERK1/2 and total ERK1/2 was quantitated using Image J software. The ratio (phosphor ERK1/2/total ERK1/2) is shown.