Figures & data
Figure 1. Defect phase diagrams as a potential new basis for materials design joining the current strategies of phase and defect engineering. An analogous picture could be drawn for Pourbaix diagrams and the interaction of corrosion mechanisms with defects.
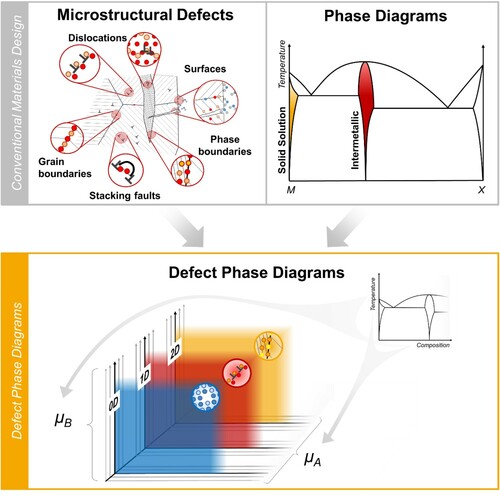
Figure 2. Defect in different states given by its chemical and structural atomic complexity. Left: ‘textbook’ symmetrical tilt boundary without chemical complexity. From middle to right: Increasing structural and chemical atomic complexity giving rise to different defect states or phases as a second atom species is added.
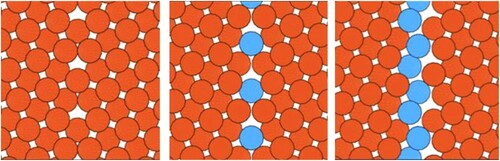
Table 1. Essential keywords in the context of defect phase diagrams.
Table 2. Definitions of suitable ‘spaces’ connecting fundamental experiments, the underlying defect phase and the resulting engineering properties.
Figure 3. Construction of defect phase diagrams using dislocations in intermetallic phases as an example. Theory and experiment work together synergistically to create defect phase diagrams.
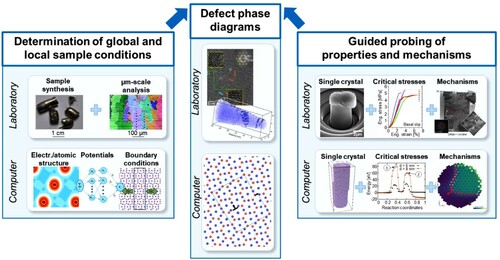
Figure 4. Continuous translation from experimental space to µ-space
to engineering space using the same example as above, dislocations in a hexagonal structure. The defect phase diagrams (DPDs) (µ-space) are connected with the mechanism maps (engineering space) via mechanism-property-diagrams (µ-space). The latter can be generated from the defect phases and their measured and computed properties and mechanisms (obtained by guided probing) to reflect a competition of choice between different mechanisms (here the competition between different slip systems and the resulting deformation behaviour).
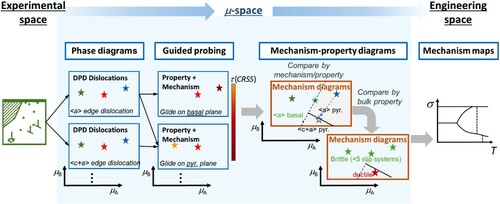
Figure 5. Visualisation of the complete framework linking all types of defect phase diagrams and defect phase properties from experimental space across µ-space to engineering space. DPD: defect phase diagram; µ: chemical potential.
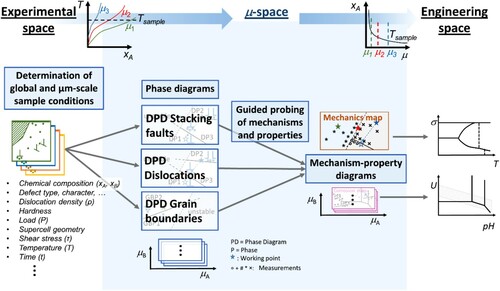
Figure 6. Schematic represerntation of the stages of defect equilibration: potential energy vs. reaction coordinate.
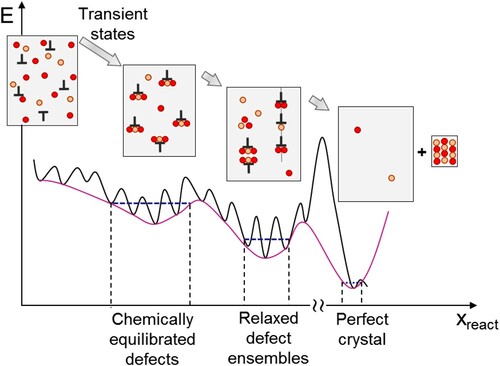
Figure 7. Selected approaches to visualise defect phase diagrams. (A) Experimental phase diagram for the Al2O3-Y2O3 system indicating stability regions of low T and high T complexions as well as their coexistence [Citation18]. Computed diagrams for effective grain boundary (GB) width (GB λ diagram) in (B) the Mo-Ni [Citation74] and (C) the W-Ni-Fe systems [Citation73]. (D) Phase diagram of the Cu-Zr system highlighting the stability of ordered (yellow) and disordered (magenta) linear complexions as function of global and local composition [Citation5]. (E) Phase diagram for the WC/Co interface where different stackings have been distinguished. The diagram is plotted as function of the chemical potential of cubic VC doping [Citation119]. Images reprinted with permission from Elsevier, Wiley and the American Physical Society from A [Citation18], B [Citation74], C [Citation73], D [Citation5], E [Citation119]. Reuse not permitted.
![Figure 7. Selected approaches to visualise defect phase diagrams. (A) Experimental phase diagram for the Al2O3-Y2O3 system indicating stability regions of low T and high T complexions as well as their coexistence [Citation18]. Computed diagrams for effective grain boundary (GB) width (GB λ diagram) in (B) the Mo-Ni [Citation74] and (C) the W-Ni-Fe systems [Citation73]. (D) Phase diagram of the Cu-Zr system highlighting the stability of ordered (yellow) and disordered (magenta) linear complexions as function of global and local composition [Citation5]. (E) Phase diagram for the WC/Co interface where different stackings have been distinguished. The diagram is plotted as function of the chemical potential of cubic VC doping [Citation119]. Images reprinted with permission from Elsevier, Wiley and the American Physical Society from A [Citation18], B [Citation74], C [Citation73], D [Citation5], E [Citation119]. Reuse not permitted.](/cms/asset/e12d0ff1-628e-4b9a-be96-47b75c87826a/yimr_a_1930734_f0007_oc.jpg)
Figure 8. Combined defect and bulk phase diagram of Ni-H. The insets show the bulk and defect phases at the atomistic scale. The data for the bulk phase diagram are taken from [Citation121] and for the defect phase diagram from [Citation120].
![Figure 8. Combined defect and bulk phase diagram of Ni-H. The insets show the bulk and defect phases at the atomistic scale. The data for the bulk phase diagram are taken from [Citation121] and for the defect phase diagram from [Citation120].](/cms/asset/0c49a1d9-0a17-43b3-9213-9d48cd2595ad/yimr_a_1930734_f0008_oc.jpg)
Figure 9. Change in the Gibbs free energy with oxygen chemical potential for Pd surfaces in contact with an oxygen atmosphere. Red lines depict the respective surface phase diagrams.
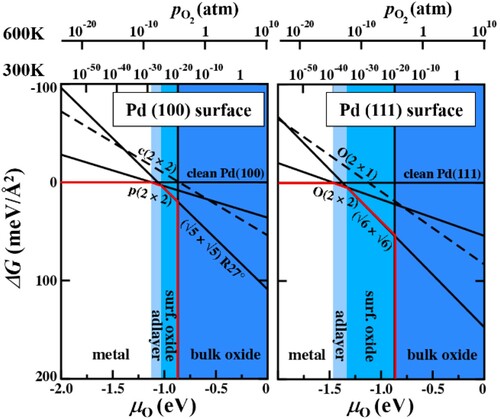
Figure 10. Surface phase diagrams for a ZnO(0001) surface in contact with a humid oxygen atmosphere. Differently coloured regions mark the stability regions of different surface reconstructions. The white dashed area indicates the region where water condensates at the surface. Geometries of the stable surface structures are shown as insets. Adapted from [Citation133] with permission from the American Physical Society. Reuse not permitted.
![Figure 10. Surface phase diagrams for a ZnO(0001) surface in contact with a humid oxygen atmosphere. Differently coloured regions mark the stability regions of different surface reconstructions. The white dashed area indicates the region where water condensates at the surface. Geometries of the stable surface structures are shown as insets. Adapted from [Citation133] with permission from the American Physical Society. Reuse not permitted.](/cms/asset/cfe2f287-9059-431d-b735-e834f801f63d/yimr_a_1930734_f0010_oc.jpg)
Figure 11. Fe–9 atomic % Mn solid solution, 50% cold-rolled and annealed at 450°C for 6 h to trigger Mn segregation. (A) Bright-field STEM image. (B) Correlative atom probe tomography results of the same tip shown in (A) using 12.5 atomic % Mn isoconcentration surfaces (12.5 atomic % Mn was chosen as a threshold value to highlight Mn-enriched regions). The blue arrows mark grain boundaries and dislocation lines that are visible in both the STEM micrograph and the atom probe tomography map. Not all dislocations visible in STEM are also visible in the atom probe data and vice versa (red arrows). (C) Overlay of (A) and (B). (D) Magnification of two subregions taken from (B). (E) 1D compositional profiles along 1 (perpendicular to dislocation line) and 2 (along dislocation line). Reprinted with permission from AAAS, Ref. [Citation3].Reuse not permitted.
![Figure 11. Fe–9 atomic % Mn solid solution, 50% cold-rolled and annealed at 450°C for 6 h to trigger Mn segregation. (A) Bright-field STEM image. (B) Correlative atom probe tomography results of the same tip shown in (A) using 12.5 atomic % Mn isoconcentration surfaces (12.5 atomic % Mn was chosen as a threshold value to highlight Mn-enriched regions). The blue arrows mark grain boundaries and dislocation lines that are visible in both the STEM micrograph and the atom probe tomography map. Not all dislocations visible in STEM are also visible in the atom probe data and vice versa (red arrows). (C) Overlay of (A) and (B). (D) Magnification of two subregions taken from (B). (E) 1D compositional profiles along 1 (perpendicular to dislocation line) and 2 (along dislocation line). Reprinted with permission from AAAS, Ref. [Citation3].Reuse not permitted.](/cms/asset/26711992-854c-485d-9d1f-bf7eba1c13e7/yimr_a_1930734_f0011_oc.jpg)
Figure 12. Atom probe tomography analysis along Mn-decorated dislocation cores, revealing low-dimensional spinodal decomposition patterns along the dislocation lines. From Kwiatkowski et al. [Citation134], published under a Creative Commons Attribution 4.0 license.
![Figure 12. Atom probe tomography analysis along Mn-decorated dislocation cores, revealing low-dimensional spinodal decomposition patterns along the dislocation lines. From Kwiatkowski et al. [Citation134], published under a Creative Commons Attribution 4.0 license.](/cms/asset/dc68c305-4191-4d13-8ef1-0e3ae145b8e8/yimr_a_1930734_f0012_oc.jpg)
Figure 13. Yttrium-similarity index, YSI for the 2850 solute pairs computed in this study and visualised in the form of a symmetric matrix (a) with yellow indicating a high similarity and blue a low one. Solute pairs that have a high index (YSI>0.95) are shown in the upper triangular part in (b). Applying a cost and solubility filter only a single pair, Al-Ca, remains (c). From [Citation141], published under a Creative Commons Attribution 4.0 International License by Springer Nature.
![Figure 13. Yttrium-similarity index, YSI for the 2850 solute pairs computed in this study and visualised in the form of a symmetric matrix (a) with yellow indicating a high similarity and blue a low one. Solute pairs that have a high index (YSI>0.95) are shown in the upper triangular part in (b). Applying a cost and solubility filter only a single pair, Al-Ca, remains (c). From [Citation141], published under a Creative Commons Attribution 4.0 International License by Springer Nature.](/cms/asset/1c153abf-eb99-4cc0-b6e7-7d39a33ce8cf/yimr_a_1930734_f0013_oc.jpg)
Figure 14. (a) Engineering stress-strain curves of the discussed Mg-Al-Ca alloy in comparison with not engineered (other than simple homogenisation treatment) solid solution Mg-Y, Mg-RE, pure Mg and Mg-Al-0.3Ca [Citation141]. The inset shows the ultimate tensile strength – uniform elongation diagram of the same alloys displaying the superior mechanical properties of the alloy developed using the YSI. RD: rolling direction; TD: transverse direction. Compositions are in weight %. (b) Pure Mg fractured along macroscopic shear bands when cold rolled to 10% thickness reduction whereas Mg–1Al–0.1Ca could be cold rolled to 54% thickness reduction in several rolling passes of 8% thickness reduction per rolling pass; small sheet sections cut from the rolled sheet after each consecutive rolling pass are presented. From [Citation141], published under a Creative Commons Attribution 4.0 International License by Springer Nature.
![Figure 14. (a) Engineering stress-strain curves of the discussed Mg-Al-Ca alloy in comparison with not engineered (other than simple homogenisation treatment) solid solution Mg-Y, Mg-RE, pure Mg and Mg-Al-0.3Ca [Citation141]. The inset shows the ultimate tensile strength – uniform elongation diagram of the same alloys displaying the superior mechanical properties of the alloy developed using the YSI. RD: rolling direction; TD: transverse direction. Compositions are in weight %. (b) Pure Mg fractured along macroscopic shear bands when cold rolled to 10% thickness reduction whereas Mg–1Al–0.1Ca could be cold rolled to 54% thickness reduction in several rolling passes of 8% thickness reduction per rolling pass; small sheet sections cut from the rolled sheet after each consecutive rolling pass are presented. From [Citation141], published under a Creative Commons Attribution 4.0 International License by Springer Nature.](/cms/asset/1aafce44-fd84-4e2c-91da-53272c97d3bd/yimr_a_1930734_f0014_oc.jpg)
Figure 15. One- and two-dimensional examples of defects with structural and chemical atomic complexity in intermetallics, metals, oxides and carbide-metal composites observed in experiments (A–D) and simulations (E–H). Images in B and F reprinted from [Citation180], published under a Creative Commons Attribution 4.0 International License. The other images are reprinted with permission from Elsevier from the following references: A [Citation154], C [Citation18], D [Citation181], E [Citation151], G [Citation18], H [Citation119]. Reuse not permitted.
![Figure 15. One- and two-dimensional examples of defects with structural and chemical atomic complexity in intermetallics, metals, oxides and carbide-metal composites observed in experiments (A–D) and simulations (E–H). Images in B and F reprinted from [Citation180], published under a Creative Commons Attribution 4.0 International License. The other images are reprinted with permission from Elsevier from the following references: A [Citation154], C [Citation18], D [Citation181], E [Citation151], G [Citation18], H [Citation119]. Reuse not permitted.](/cms/asset/7188d1e5-e46b-4321-8f8e-10242a6cc5cb/yimr_a_1930734_f0015_oc.jpg)
Figure 16. Combination of experimental and computation nanomechanics enables scale-bridging study of deformation mechanisms in ordered crystals, which tend to be brittle at the macroscopic scale and low temperature, enabling the population of defect phase and mechanism-property diagrams in the future. Measurement of critical stresses for yielding (A) and energy barriers for different deformation mechanisms (F) from experimental (C) and computational (D) nanomechanical studies. Observation of both static and moving dislocations at the same level of resolution is made possible by HR-TEM (B) and molecular dynamics simulations (E). Images reprinted with permission from Elsevier and Wiley from references A [Citation168], B [Citation174], C [Citation182], D [Citation156], E [Citation183], F [Citation156]. Reuse not permitted.
![Figure 16. Combination of experimental and computation nanomechanics enables scale-bridging study of deformation mechanisms in ordered crystals, which tend to be brittle at the macroscopic scale and low temperature, enabling the population of defect phase and mechanism-property diagrams in the future. Measurement of critical stresses for yielding (A) and energy barriers for different deformation mechanisms (F) from experimental (C) and computational (D) nanomechanical studies. Observation of both static and moving dislocations at the same level of resolution is made possible by HR-TEM (B) and molecular dynamics simulations (E). Images reprinted with permission from Elsevier and Wiley from references A [Citation168], B [Citation174], C [Citation182], D [Citation156], E [Citation183], F [Citation156]. Reuse not permitted.](/cms/asset/337553d7-acae-49ae-b4cc-00dcbba7cc90/yimr_a_1930734_f0016_oc.jpg)
Figure 17. (a) Atomic structures and energy surface for the tensile twins in pure Mg. (b) Visualisation of the shear parameter
in units of half the Burgers vector
and the shuffle parameter
in units of the atomic plane distance
describing the state of the TB. The energy minima correspond to the reflection TB (
) and the glide TB (
). The energies have been determined by an EAM potential. Figure adapted from Ref. [Citation175], published under a Creative Commons Attribution 4.0 International License by Springer Nature.
![Figure 17. (a) Atomic structures and energy surface for the {101¯2} tensile twins in pure Mg. (b) Visualisation of the shear parameter ϵ in units of half the Burgers vector b and the shuffle parameter s in units of the atomic plane distance d describing the state of the TB. The energy minima correspond to the reflection TB (ϵ=0,s=0) and the glide TB (ϵ=1,s=1). The energies have been determined by an EAM potential. Figure adapted from Ref. [Citation175], published under a Creative Commons Attribution 4.0 International License by Springer Nature.](/cms/asset/ea1a1200-a801-4c4b-ab68-568177681857/yimr_a_1930734_f0017_oc.jpg)
Figure 18. (a) and (b) show the DOS available to dilutely segregating Mg atoms at (130) and (430) [001]5 Al GBs, respectively, calculated at 0 K with an empirical potential. Yellow (red) curves show Fermi-Dirac occupation of these states at 100 K (700 K). (c) and (d) ((e) and (f)) show the resulting structure predictions for these boundaries at low (high) temperature. Structures are projected onto the (001) plane and coloured according to Mg occupation probability (Al = grey, Mg = blue). For the disorderly (430) boundary, translucency is used to indicate atomic density in the [001] direction.
![Figure 18. (a) and (b) show the DOS available to dilutely segregating Mg atoms at (130) and (430) [001]Σ5 Al GBs, respectively, calculated at 0 K with an empirical potential. Yellow (red) curves show Fermi-Dirac occupation of these states at 100 K (700 K). (c) and (d) ((e) and (f)) show the resulting structure predictions for these boundaries at low (high) temperature. Structures are projected onto the (001) plane and coloured according to Mg occupation probability (Al = grey, Mg = blue). For the disorderly (430) boundary, translucency is used to indicate atomic density in the [001] direction.](/cms/asset/957d8e83-a20a-4a04-92f8-262e1cc59cdd/yimr_a_1930734_f0018_oc.jpg)
Figure 19. Coupled Molecular Dynamics/Monte Carlo simulations for Al-Mg at room temperature for increasing time/Monte Carlo steps. Positions are projected onto the (001) plane and grouped by column, with colour indicating species (grey = Al, blue = Mg). Red and yellow circles indicate areas of special interest (see text). Figure adapted from [Citation179], published under a Creative Commons International 4.0 Attribution license.
![Figure 19. Coupled Molecular Dynamics/Monte Carlo simulations for Al-Mg at room temperature for increasing time/Monte Carlo steps. Positions are projected onto the (001) plane and grouped by column, with colour indicating species (grey = Al, blue = Mg). Red and yellow circles indicate areas of special interest (see text). Figure adapted from [Citation179], published under a Creative Commons International 4.0 Attribution license.](/cms/asset/0a52159c-0040-4874-848f-8bbf173f5546/yimr_a_1930734_f0019_oc.jpg)
