Figures & data
Figure 1. The study flowchart. The full analysis set (FAS) included all patients who underwent randomization, received at least one dose of rhTPO, and had a baseline assessment and at least one post-baseline assessment. The per protocol set (PPS) included patients who met the eligibility criteria and completed the treatment as specified by the trial protocol and completed efficacy evaluation on day 14. The safety set included all patients who received at least one dose of rhTPO and had at least one follow-up safety assessment and was analyzed mainly using descriptive statistics. rhTPO, recombinant human thrombopoietin.
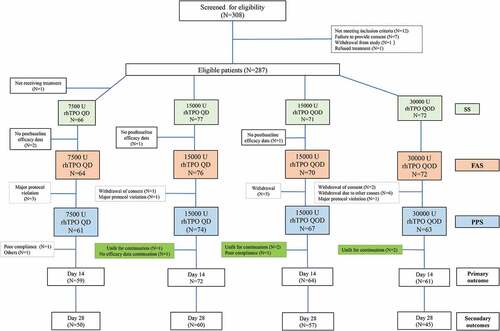
Table I. Patient demographic and baseline characteristics-the full analysis set (FAS).
Figure 2. Median platelet count over time (a) and median change in platelet count (b) from baseline on day 7, 14 and 28 in the FAS. Platelet count changes from baseline were analyzed using the analysis of covariance (ANCOVA) in which the baseline platelet count was used as a covariate, groups as a fixed effect, and centers as a random effect. ^day 7, P = .027; *day 14, P < .001; *day 28, P = .388.
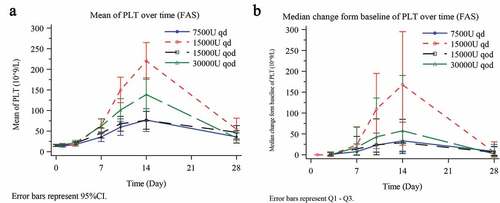
Table II. Primary and secondary efficacy outcomes in the full analysis set (FAS).
Table III. Adverse events in the safety set.
