Figures & data
Figure 1. Cellular components from L. rhamnosus MLGA-induced AvBD9 mRNA expression in primary chicken intestinal epithelial cells. (A) Intestinal epithelial cells were incubated with five different components at the indicated concentrations for 4 h each. (B) Intestinal epithelial cells were stimulated with WPG at a concentration of 50 µg/mL for 2, 4, 6, 8, and 12 h. AvBD9 mRNA expression was determined by qPCR. Values are given as the mean ± SD. n = 3 for each treatment. S: cultural supernatant of L. rhamnousus; CWP: cell wall protein; LTA: lipoteichoic acid; WCW: whole cell wall; WPG: whole peptidoglycan.
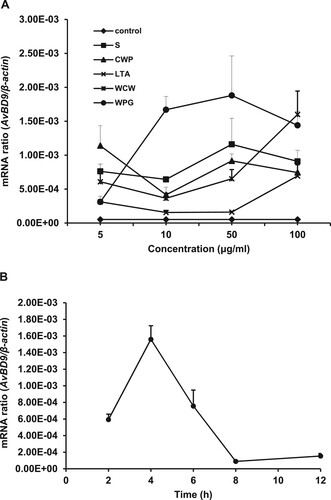
Figure 2. Role of TLR2 in the induction of L. rhamnosus MLGA-dependent AvBD9 mRNA expression in primary chicken intestinal epithelial cells. (A) Upregulation of TLR2 mRNA expression by L. rhamnosus MLGA preparations. Intestinal epithelial cells were treated with live or heat-killed L. rhamnosus MLGA at a concentration of 2 × 106 CFU/mL or WPG at a concentration of 50 µg/mL for 4 h. TLR2 mRNA expression was determined by qPCR. Values are given as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared with the control group. (B) Blocking TLR2 inhibited AvBD9 mRNA expression induced by L. rhamnosus MLGA preparations. Intestinal epithelial cells were pretreated with 10 µg/mL anti-TLR2 antibody for 40 min and then stimulated with live or heat-killed L. rhamnosus MLGA or WPG. **P < 0.01 between the indicated treatments; #P < 0.05, ##P < 0.01 compared with control group. Values are given as the mean ± SD of three independent experiments.
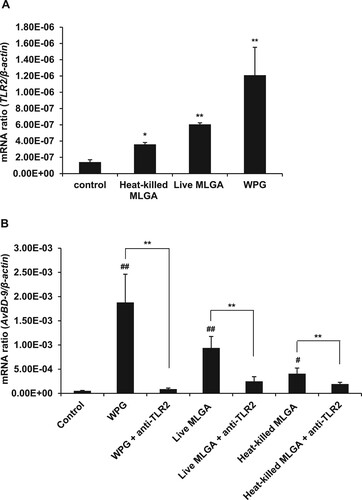
Figure 3. EMSA analysis of DNA-binding activities of NF-κB and AP-1 in chicken intestinal epithelial cells. Cells were stimulated with L. rhamnosus MLGA or WPG for 4 h. NF-κB and AP-1 DNA-binding activities in the nuclear extracts were assessed by EMSA. EMSA results demonstrate that NF-κB (A) and AP-1 (B) DNA-binding activities were increased by stimulation with WPG, live L. rhamnosus MLGA, or heat-killed L. rhamnosus MLGA, with WPG-treated cells being strongly positive. Lane FP: the free probe only control; lane -: negative control without nuclear protein; lane +: positive control with nuclear proteins from Mo7e cells; lane WPG: nuclear extracts from WPG-treated cells; lane L-M: nuclear extracts from live L. rhamnosus MLGA-treated cells; lane H-M: nuclear extracts from heat-killed L. rhamnosus MLGA-treated cells; lane Con: nuclear extracts from untreated control cells. Data are representative of three independent experiments.
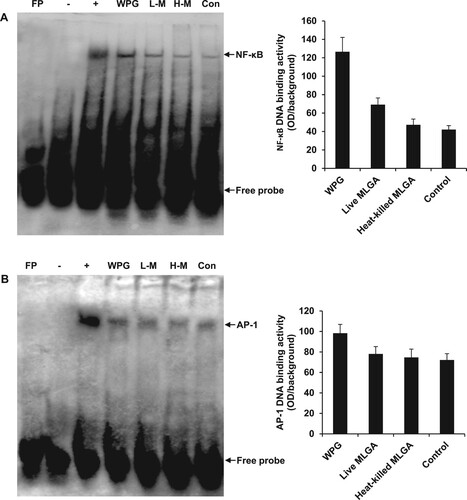
Figure 4. Induction of AvBD9 in intestinal epithelial cells by L. rhamnosus MLGA and WPG is dependent on NF-κB or JNK, but not ERK1/2 and p38 MAPK pathways. PDTC, an inhibitor of NF-κB, completely inhibited AvBD9 expression induced by WPG (A), live L. rhamnosus MLGA (B), and heat-killed L. rhamnosus MLGA (C) in intestinal epithelial cells. SP600125, an inhibitor of JNK, potentially inhibited WPG- (A), live L. rhamnosus MLGA- (B), and heat-killed L. rhamnosus MLGA (C)-induced AvBD9 expression in intestinal epithelial cells, whereas p38 MAPK inhibitor SB203580 and ERK 1/2 inhibitor PD98059 showed little or no effect. Intestinal epithelial cells were pretreated with PDTC (50 μM), SP600125 (20 μM), SB203580 (20 μM), and PD98059 (20 μM) for 1 h and then treated with L. rhamnosus MLGA preparations for 4 h. AvBD9 mRNA expression was determined by qPCR. NS, not significant between the indicated treatments, **P < 0.01 between the indicated treatments. Values are given as the mean ± SD of three independent experiments.
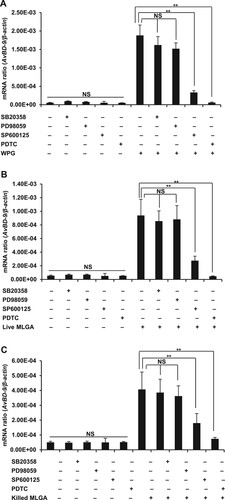
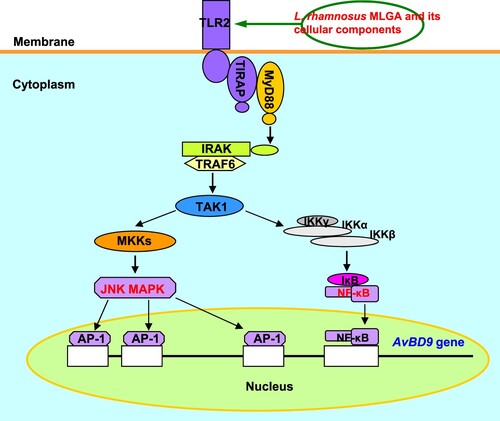
Figure 5. Schematic diagram of the role of TLR2-mediated signalling in AvBD9 induction in chicken intestinal epithelial cells by L. rhamnosus MLGA and its whole cell wall peptidoglycan. The NF-κB and JNK MAPK signalling pathways are activated by TLR2 stimulation with L. rhamnosus MLGA or its whole cell wall peptidoglycan, leading to the induction of AvBD9 expression.