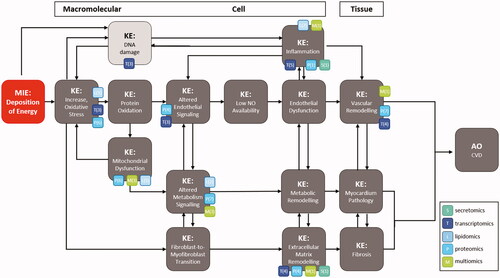
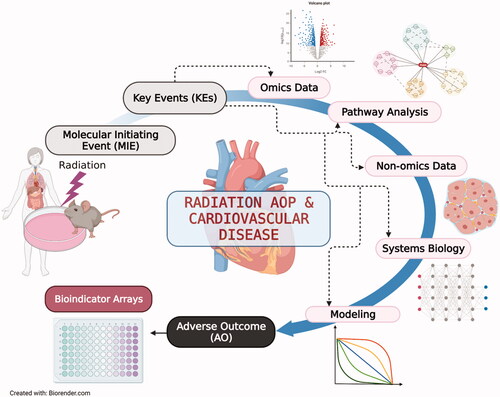
Figures & data
Table 1. List of the transcriptomic studies on radiation-induced CVD.
Table 2. List of the miRNA analyses of radiation-induced CVD.
Table 3. List of the proteomics studies on radiation-induced CVD.
Table 4. List of the metabolomics studies on radiation-induced CVD.
Table 5. List of the biofluid analyses on radiation-induced CVD.