Figures & data
Table 1. The results of crossing experiments carried out with different strains of Fragilariopsis kerguelensis; in the first column and in the first row the strain code, in the second column the mating type (m.t., see text for further details), in the third column the average apical length of the strains (Size). Grey-filled squares indicate the absence of intraclonal sexual reproduction; + indicates the presence of sexual stages; 0 indicates the absence of sexual stages; n.a. = not available.
Figs 1–13. Light (Figs 1–4) and SEM (Figs 5–13) micrographs of Fragilariopsis kerguelensis vegetative cells. 1–3. Cells of different apical length arranged in chains; an arrowhead points to the nucleus (Fig. 1, strain PA_P6C3; Fig. 2, strain MM_P7A4; Fig. 3, strain MM_E8A6). 4. A single cell in valvar view (strain MM_E13C5). 5. A detail of a chain in which mucous material is visible between the valves of adjacent cells (large F1 generation cells of cross PA_P8B1 × MM_P13D2, experiment B). 6. An isopolar valve; the narrow raphe on the valve margin is arrowed (natural sample). 7. Epi- and hypotheca of a dividing cell showing the valvocopula and the two cingular bands; the diagonal suture in the valvocopula is marked with an arrow and the ligula on the second, thin cingular band is marked with arrowheads (cross PA_P8B1 × MM_P13D2, experiment B). 8. A detail of Fig. 7. 9. Epitheca showing valvocopula and two cingular bands; note the diagonal suture in the valvocopula (arrow) and the ligula on the narrow second cingular band (arrowhead) (cross PA_P8B1 × MM_P13D2, experiment B). 10. Detail of Fig. 9. 11. A heteropolar valve (strain Lynn 5). 12. Detail of the valve with the poroids (strain Lynn 5). 13. Detail of the same valve, showing a single poroid with minute perforations. Scale bars = 10 µm (Figs 1–5, 7, 11), 5 µm (Figs 6, 8, 9), 1 µm (Figs 10, 12) and 100 nm (Fig. 13).
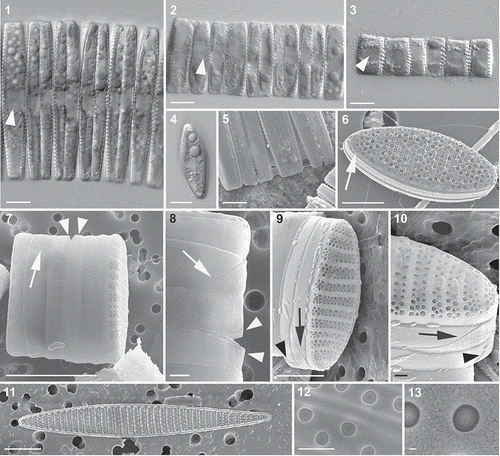
Table 2. Rate of reduction in cell size (apical length) and growth rate for seven Fragilariopsis kerguelensis strains. The cell size is the average cell size on day 0 and the growth rate is the average of three measurements.
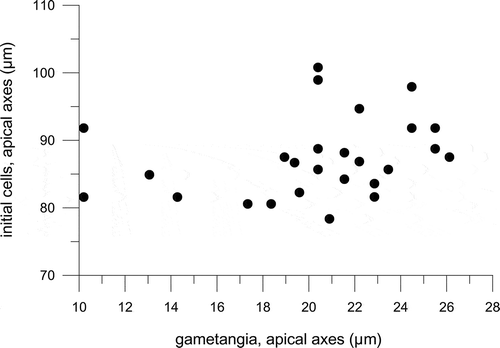
Fig. 14. Average monthly reduction rate of the apical length of Fragilariopsis kerguelensis clonal strains of different cell size; black circles represent the 47 strains measured after a time interval of 24 months, white circles represent the seven strains measured after a time interval of 7 months.
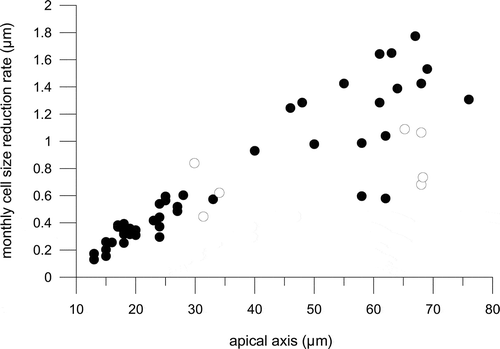
Fig. 15. Percentage distribution of single cells (grids), cells in chains (white) and sexual stages (black) in monoclonal parental strains (L2-D6 and L9-C3) of Fragilariopsis kerguelensis and in three replicate crosses on day 7 after inoculation.
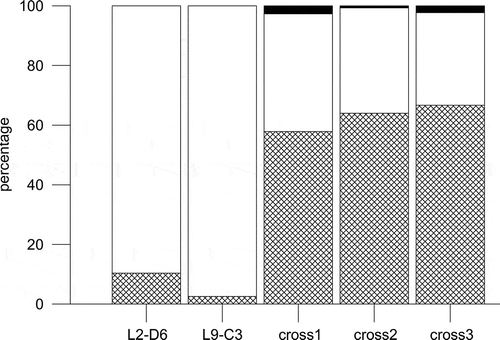
Figs 16–22. Light (Figs 16, 17) and SEM (Figs 18–22) micrographs of gametangia of Fragilariopsis kerguelensis. 16. A few days after the inoculation together of strains of opposite mating type: cells have detached from chains and several cells are in contact (arrows) (mixture of different strains in experiment A). 17. Picture taken on the same day as , where single cells in girdle view have an enlarged nucleus (arrowhead) and chloroplasts appressed to the valves (experiment A). 18. Two cells (gametangia) close to and perpendicular to each other, in contact via the cingulum (cross PA_P8B1 × MM_P13D2, experiment B). 19. Detail of the same pair of cells showing the mucous material extruded from the girdle region that keeps the two adjacent cells together. 20. A gametangium in girdle view with extra cingular bands in the hypotheca (arrows) (cross L2D6 × L9C3, experiment C). 21. Gametangium with extra cingular bands in the hypotheca (cross PA_P8B1 × MM_P13D2, experiment B). 22. An auxospore and three gametangial hypothecae with extra cingular bands (cross L2D6 × L9C3, experiment C). Scale bars = 20 µm (, ), 5 µm (, –) and 1 µm ().
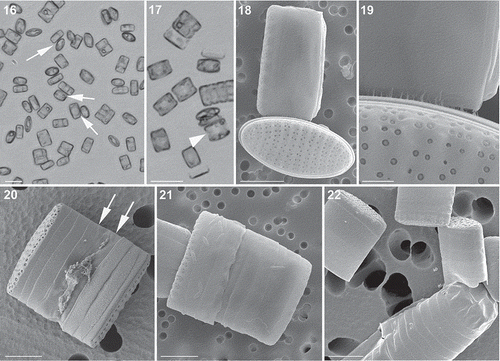
Figs 23–41. Light and epifluorescence micrographs of auxosporulation in Fragilariopsis kerguelensis. 23. Two appressed gametangia in girdle view; the cell on the right shows the cleavage of cytoplasm (cytokinesis) (cross L2D6 × L9C3, experiment C). 24. The same gametangia with DAPI-stained nuclei; the cell on the left has an expanded nucleus in meiotic prophase, the cell on the right has undergone the second meiotic division and each protoplast has two nuclei. 25. A gametangium after completion of cytokinesis and the formation of two gametes (cross L2D6 × L9C3, experiment C). 26. The same gametangium with DAPI-stained nuclei; each gamete has two nuclei. 27. A zygote still attached to a theca of the gametangium (cross L2D6 × L9C3, experiment C). 28. The same zygote with four DAPI-stained nuclei (arrowheads). 29. A zygote still attached to a theca of the gametangium (cross PA_P8B1 × MM_P13D2, experiment B). 30. The same zygote stained with PDMPO, showing thin siliceous platelets in its wall (arrowheads). 31. A young auxospore still attached to one theca of a gametangium (cross PA_P8B1 × MM_P13D2, experiment B). 32. The same auxospore stained with PDMPO, showing thin siliceous platelets at both ends of the auxospore (arrowheads). 33. An elongating auxospore still attached to one theca of a gametangium (mix of different strains in experiment A). 34. The same auxospore with two DAPI-stained nuclei. 35. An elongated auxospore still attached to the theca of a gametangium; mix of different strains in experiment A). 36. The same auxospore stained with PDMPO, showing the bands of the transverse perizonium. 37. An elongated auxospore still attached to the theca of a gametangium (mix of different strains in experiment A). 38. The same auxospore stained with PDMPO, showing the terminal bands of the transverse perizonium (arrowheads) and the longitudinal perizonium. 39. An elongated auxospore; the sample was incubated with PDMPO and the internal longitudinal perizonium, the thin bands of the transversal perizonium and the terminal caps (arrowheads) are visible (cross PA_P8B1 × MM_P13D2, experiment B). 40. A mature auxospore within which one valve of the initial cell has been deposited (mix of different strains in experiment A). 41. The same auxospore with two DAPI-stained nuclei and PDMPO-stained valve (on the top side). Scale bars = 10 µm.
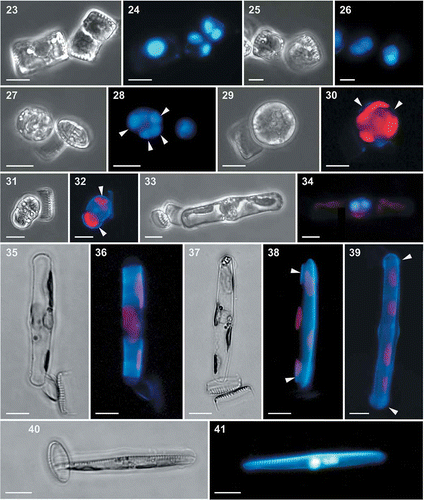
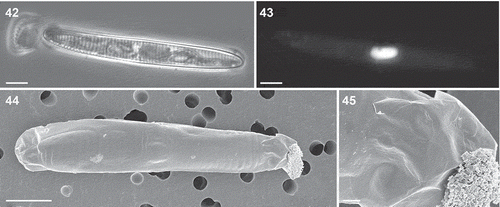
Figs 42–45. Auxospores of Fragilariopsis kerguelensis. 42. Light micrograph of a mature auxospore containing the initial cell (mix of different strains in experiment A). 43. Epifluorescence micrograph of the same auxospore showing the fusion of the two DAPI-stained nuclei. 44. SEM micrograph of an auxospore (cross L2D6 × L9C3, experiment C), showing caps at the ends and the transverse perizonium (the faint rings around the auxospore: cross L2D6 × L9C3, experiment C). 45. A detail of showing round scales covering the terminal portion of the auxospore. Scale bars = 10 µm (Figs 42–44) and 1 µm ().