Figures & data
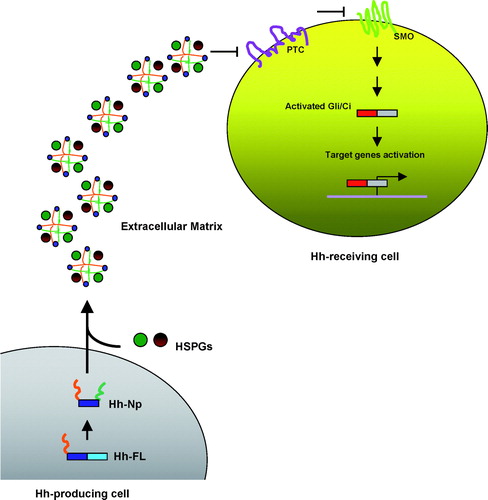
Figure 1. MBOAT family members’ topology. MBOAT (membrane-bound O-acyltransferase) family members that promote protein acylation – Hhat, Porc and GOAT – contain multiple membrane-spanning domains. These palmitoylacyltransferases (PATs) are responsible for attaching fatty acids (originating in the cytoplasm in the form of activated CoA esters) to secreted signalling proteins (SSP), such as Hedgehogs, Wg/Wnts and ghrelin, within the lumen of the secretory pathway. The histidine shown is conserved in almost all members of the MBOAT family and may contribute to the active site of the putative acyltransferase. This Figure is reproduced in colour in Molecular Membrane Biology online.
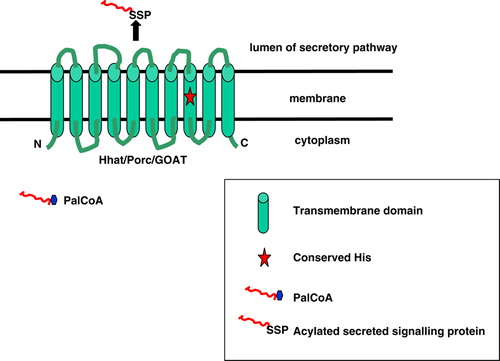
Figure 2. Post-translational modifications of Hh. Drosophila and vertebrate Hedgehog proteins are synthesized as ∼45 kDa precursors that undergo dual lipidation as well as internal autoprocessing at a conserved sequence. After its signal peptide is removed co-translationally, the precursor is palmitoylated at its N-terminal cysteine using PalmitoylCoA. Palmitoylation is facilitated by Hhat and it is suggested that an intramolecular S-to-N acyl shift occurs, transferring the thioester-linked fatty acid to amide linkage. Autocatalytic cleavage occurs at the GCF sequence, catalyzed by the C-terminal domain of the precursor, and produces a ∼19 kDa signalling domain Hh-N and a ∼25 kDa Hh-C. During the autocleavage event, Hh-N is modified by cholesterol at its carboxy-terminal glycine to form a fully active Hh-Np which contains all known signalling activities. This Figure is reproduced in colour in Molecular Membrane Biology online.
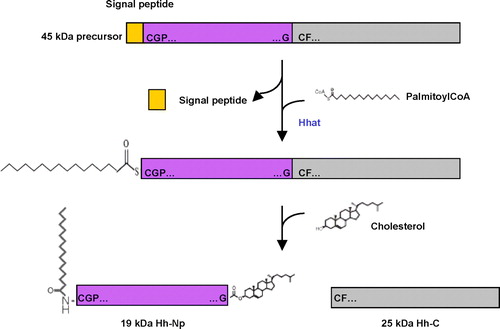
Figure 3. Model of active soluble Hh/Shh multimeric complex transport. After post-translational modifications in Hh-producing cells (palmitoylation, red; cholesteroylation, green), Hh-Np proteins are secreted to the extracellular matrix and form a multimeric complex with Heparan Sulphate Proteoglycans (HSPGs). In Hh-receiving cells, the receptor PTC is repressed by Hh and this releases SMO to activate downstream signalling. HSPGs can improve the solubility of Hh proteins during intercellular trafficking in the extracellular matrix and enhance Hh-PTC interaction on Hh-receiving cells, therefore promoting subsequent signalling activity.