Figures & data
Figure 1. Effect of cationic lipid/DNA (+/-) charge ratio, PEI/DNA ratio [nitrogen/phosphate (N/P)] (2.7 KDa PEI synthesized in the laboratory) of PEI-Tf-complexes on β-galactosidase (β-gal) gene expression in HeLa cells. PEI-Tf-complexes were obtained from DOTAP:Chol liposomes prepared by the hydration-extrusion method, as described in Materials and methods. The liposomes were complexed, at the indicated lipid/DNA charge ratios, with 1 µg of plasmid DNA, pre-condensed with 2.7 KDa PEI, at the indicated PEI/DNA in the presence of transferrin (32 µg/µg DNA). After 4 h incubation of PEI-Tf-complexes with HeLa cells in DMEM-HG containing 10% FBS, the medium was replaced with fresh medium also in the presence of 10% FBS. The cells were further incubated for 48 h and the levels of gene expression were evaluated as described in Materials and methods. The data are expressed as µg of β-gal per mg of total cell protein (mean±SEM obtained from triplicates) and are representative of, at least, three independent experiments.
![Figure 1. Effect of cationic lipid/DNA (+/-) charge ratio, PEI/DNA ratio [nitrogen/phosphate (N/P)] (2.7 KDa PEI synthesized in the laboratory) of PEI-Tf-complexes on β-galactosidase (β-gal) gene expression in HeLa cells. PEI-Tf-complexes were obtained from DOTAP:Chol liposomes prepared by the hydration-extrusion method, as described in Materials and methods. The liposomes were complexed, at the indicated lipid/DNA charge ratios, with 1 µg of plasmid DNA, pre-condensed with 2.7 KDa PEI, at the indicated PEI/DNA in the presence of transferrin (32 µg/µg DNA). After 4 h incubation of PEI-Tf-complexes with HeLa cells in DMEM-HG containing 10% FBS, the medium was replaced with fresh medium also in the presence of 10% FBS. The cells were further incubated for 48 h and the levels of gene expression were evaluated as described in Materials and methods. The data are expressed as µg of β-gal per mg of total cell protein (mean±SEM obtained from triplicates) and are representative of, at least, three independent experiments.](/cms/asset/63c0d18b-0014-4adf-b855-a815e515f43b/imbc_a_376841_f0001_b.gif)
Figure 2. Effect of the lipid composition, cationic lipid/DNA (+/-) charge ratio, PEI/DNA ratio [nitrogen/phosphate (N/P)] (2.7 KDa PEI synthesized in the laboratory) of PEI-Tf-complexes on β-galactosidase (β-gal) gene expression in HeLa cells. PEI-Tf-complexes were obtained from liposomes (DOTAP:Chol or DOTAP:CHEMS:DOPE:Chol) prepared by the ethanol injection method, in the presence or absence of the detergent octyl β-D-glucopyranoside (OGP), as described in Materials and methods. Experiments were performed as described in the legend to . The PEI-Tf-complexes were produced from (A) DOTAP:Chol liposomes prepared in the absence of OGP (DC); (B) DOTAP:Chol liposomes prepared in the presence of 20 mM OGP (DC-det); (C) DOTAP:CHEMS:DOPE:Chol liposomes (CatpH) and (D) DOTAP:CHEMS:DOPE:Chol liposomes prepared in the presence of 20 mM OGP (CatpH-det). The data are expressed as µg of β-gal per mg of total cell protein (mean±SEM obtained from triplicates) and are representative of, at least, three independent experiments.
![Figure 2. Effect of the lipid composition, cationic lipid/DNA (+/-) charge ratio, PEI/DNA ratio [nitrogen/phosphate (N/P)] (2.7 KDa PEI synthesized in the laboratory) of PEI-Tf-complexes on β-galactosidase (β-gal) gene expression in HeLa cells. PEI-Tf-complexes were obtained from liposomes (DOTAP:Chol or DOTAP:CHEMS:DOPE:Chol) prepared by the ethanol injection method, in the presence or absence of the detergent octyl β-D-glucopyranoside (OGP), as described in Materials and methods. Experiments were performed as described in the legend to Figure 1. The PEI-Tf-complexes were produced from (A) DOTAP:Chol liposomes prepared in the absence of OGP (DC); (B) DOTAP:Chol liposomes prepared in the presence of 20 mM OGP (DC-det); (C) DOTAP:CHEMS:DOPE:Chol liposomes (CatpH) and (D) DOTAP:CHEMS:DOPE:Chol liposomes prepared in the presence of 20 mM OGP (CatpH-det). The data are expressed as µg of β-gal per mg of total cell protein (mean±SEM obtained from triplicates) and are representative of, at least, three independent experiments.](/cms/asset/c16371a7-712f-492c-b5ae-776104ade108/imbc_a_376841_f0002_b.gif)
Figure 3. Effect of the N/P ratio and molecular weight of PEI on its capacity to complex DNA. 1 µg of DNA was complexed with PEI (0.8 or 2 KDa) at the indicated N/P ratios in the presence of ethidium bromide (EtBr), as described in Materials and methods. The amount of DNA available to interact with EtBr was calculated by subtracting the values of residual fluorescence from those obtained for the samples and expressed as the percentage of the control. Control corresponds to the fluorescence of the probe in the presence of 1 µg DNA (100% of EtBr accessibility). The results correspond to the mean±SEM obtained from two independent experiments.
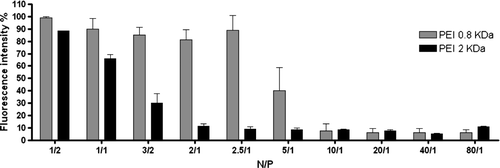
Figure 4. Effect of the lipid composition, cationic lipid/DNA (+/-) charge ratio, PEI/DNA ratio [nitrogen/phosphate (N/P)] (2.0 KDa PEI) of PEI-Tf-complexes on β-galactosidase (β-gal) gene expression in HeLa cells. PEI-Tf-complexes exhibiting the highest levels of transfection when prepared with 2.7 KDa PEI (Figure 2) were selected to further test their transfection activity upon pre-condensation of the plasmid DNA with commercialized 2.0 KDa PEI. ]Preparation of the PEI-Tf-complexes, at the indicated lipid/DNA charge ratios and PEI/DNA ratios, and transfection experiments were carried out as described in the legend to . (A) DOTAP:Chol liposomes prepared in the absence of OGP (DC); (B) DOTAP:Chol liposomes prepared in the presence of 20 mM OGP (DC-det); (C) DOTAP:CHEMS:DOPE:Chol liposomes prepared in the absence of OGP (CatpH) and (D) DOTAP:CHEMS:DOPE:Chol liposomes prepared in the presence of 20 mM OGP (CatpH-det). The data are expressed as µg of β-gal per mg of total cell protein (mean±SEM obtained from triplicates) and are representative of, at least, three independent experiments.
![Figure 4. Effect of the lipid composition, cationic lipid/DNA (+/-) charge ratio, PEI/DNA ratio [nitrogen/phosphate (N/P)] (2.0 KDa PEI) of PEI-Tf-complexes on β-galactosidase (β-gal) gene expression in HeLa cells. PEI-Tf-complexes exhibiting the highest levels of transfection when prepared with 2.7 KDa PEI (Figure 2) were selected to further test their transfection activity upon pre-condensation of the plasmid DNA with commercialized 2.0 KDa PEI. ]Preparation of the PEI-Tf-complexes, at the indicated lipid/DNA charge ratios and PEI/DNA ratios, and transfection experiments were carried out as described in the legend to Figure 1. (A) DOTAP:Chol liposomes prepared in the absence of OGP (DC); (B) DOTAP:Chol liposomes prepared in the presence of 20 mM OGP (DC-det); (C) DOTAP:CHEMS:DOPE:Chol liposomes prepared in the absence of OGP (CatpH) and (D) DOTAP:CHEMS:DOPE:Chol liposomes prepared in the presence of 20 mM OGP (CatpH-det). The data are expressed as µg of β-gal per mg of total cell protein (mean±SEM obtained from triplicates) and are representative of, at least, three independent experiments.](/cms/asset/d1264175-5ed5-4e86-9de1-b014e07c325f/imbc_a_376841_f0004_b.gif)
Figure 5. Effect of the lipid composition, cationic lipid/DNA (+/-) charge ratio, PEI/DNA ratio [nitrogen/phosphate (N/P)] (0.8 KDa PEI) of PEI-Tf-complexes on β-galactosidase (β-gal) gene expression in HeLa cells. PEI-Tf-complexes exhibiting the highest levels of transfection when prepared with 2.7 KDa PEI (Figure 2) were selected to further test their transfection activity upon pre-condensation of the plasmid DNA with the commercialized 0.8 KDa PEI. Preparation of the PEI-Tf-complexes, at the indicated lipid/DNA charge ratios and PEI/DNA ratios, and transfection were carried out as described in the legend to . (A) DOTAP:Chol liposomes prepared in the absence of OGP (DC); (B) DOTAP:Chol liposomes prepared in the presence of 20 mM OGP (DC-det), (C) DOTAP:CHEMS:DOPE:Chol liposomes prepared in the absence of OGP (CatpH) and (D) DOTAP:CHEMS:DOPE:Chol liposomes prepared in the presence of 20 mM OGP (CatpH-det). The data are expressed as µg of β-gal per mg of total cell protein (mean±SEM obtained from triplicates) and are representative of, at least, three independent experiments.
![Figure 5. Effect of the lipid composition, cationic lipid/DNA (+/-) charge ratio, PEI/DNA ratio [nitrogen/phosphate (N/P)] (0.8 KDa PEI) of PEI-Tf-complexes on β-galactosidase (β-gal) gene expression in HeLa cells. PEI-Tf-complexes exhibiting the highest levels of transfection when prepared with 2.7 KDa PEI (Figure 2) were selected to further test their transfection activity upon pre-condensation of the plasmid DNA with the commercialized 0.8 KDa PEI. Preparation of the PEI-Tf-complexes, at the indicated lipid/DNA charge ratios and PEI/DNA ratios, and transfection were carried out as described in the legend to Figure 1. (A) DOTAP:Chol liposomes prepared in the absence of OGP (DC); (B) DOTAP:Chol liposomes prepared in the presence of 20 mM OGP (DC-det), (C) DOTAP:CHEMS:DOPE:Chol liposomes prepared in the absence of OGP (CatpH) and (D) DOTAP:CHEMS:DOPE:Chol liposomes prepared in the presence of 20 mM OGP (CatpH-det). The data are expressed as µg of β-gal per mg of total cell protein (mean±SEM obtained from triplicates) and are representative of, at least, three independent experiments.](/cms/asset/df0f6bb9-7862-4d54-9e7f-fe5c4512ce74/imbc_a_376841_f0005_b.gif)
Figure 6. Effect of the lipid composition, cationic lipid/DNA (+/-) charge ratio, PEI/DNA ratio [nitrogen/phosphate (N/P)] (2.0 KDa PEI) of PEI-complexes on β-galactosidase (β-gal) gene expression in HeLa cells. The most efficient liposome formulations using 2.0 KDa PEI (Figure 4) were selected to examine the effect of transferrin on transfection. PEI-complexes lacking transferrin were obtained from liposomes containing DOTAP:Chol (DC) or DOTAP:CHEMS:DOPE:Chol with OGP (CatpH-det) prepared by the ethanol injection method, as described in Materials and methods. Transfection was carried out as described in the legend to . (A) DOTAP:Chol liposomes (DC), (B) DOTAP:CHEMS:DOPE:Chol liposomes prepared in the presence of 20 mM OGP (CatpH-det). The data are expressed as µg of β-gal per mg of total cell protein (mean±SEM obtained from triplicates) and are representative of, at least, three independent experiments.
![Figure 6. Effect of the lipid composition, cationic lipid/DNA (+/-) charge ratio, PEI/DNA ratio [nitrogen/phosphate (N/P)] (2.0 KDa PEI) of PEI-complexes on β-galactosidase (β-gal) gene expression in HeLa cells. The most efficient liposome formulations using 2.0 KDa PEI (Figure 4) were selected to examine the effect of transferrin on transfection. PEI-complexes lacking transferrin were obtained from liposomes containing DOTAP:Chol (DC) or DOTAP:CHEMS:DOPE:Chol with OGP (CatpH-det) prepared by the ethanol injection method, as described in Materials and methods. Transfection was carried out as described in the legend to Figure 1. (A) DOTAP:Chol liposomes (DC), (B) DOTAP:CHEMS:DOPE:Chol liposomes prepared in the presence of 20 mM OGP (CatpH-det). The data are expressed as µg of β-gal per mg of total cell protein (mean±SEM obtained from triplicates) and are representative of, at least, three independent experiments.](/cms/asset/7abca562-6da5-4e58-a8f8-c95c93709aaa/imbc_a_376841_f0006_b.gif)
Figure 7. Effect of cationic lipid/DNA charge ratio (+/-), N/P ratio and liposome formulation on the access of ethidium bromide to DNA associated with the PEI-complexes (2.0 KDa PEI) in the presence or absence of transferrin. PEI-complexes, containing 1 µg of DNA, were obtained from DOTAP:Chol liposomes (DC) associated with (A) or without (B) transferrin, or from DOTAP:CHEMS:DOPE:Chol liposomes containing OGP (CatpH-det) associated with transferrin (C), prepared by the ethanol injection method. Complexes were incubated with EtBr as described in Materials and methods. The amount of DNA available to interact with the probe was calculated by subtracting the values of residual fluorescence from those obtained for the samples and expressed as the percentage of the control. Control corresponds to the fluorescence of the probe in the presence of 1 µg DNA (100% of EtBr accessibility). The results correspond to the mean±SEM obtained from triplicates, and are representative of two independent experiments.
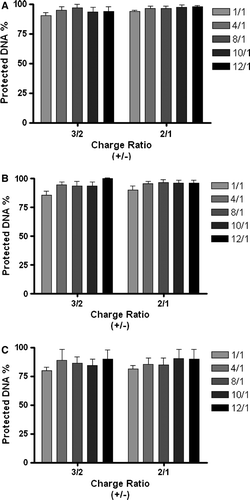
Figure 8. Effect of lipid/DNA (+/-) charge ratio, N/P ratio and lipid composition on the extent of association of PEI-complexes (2.0 KDa PEI) with HeLa cells. PEI-complexes were obtained from DOTAP:Chol liposomes (DC), associated with (A) or without (B) transferrin, or DOTAP:CHEMS:DOPE:Chol liposomes containing OGP (CatpH-det) and associated with transferrin (C), prepared by the ethanol injection method. Liposomes were labeled by incorporating Rh-PE into the lipid bilayer, at a concentration of 5 mol% of total lipid, and the extent of cell association was measured after 4 h incubation at 37°C of the complexes with the cells, as described in Materials and methods. The data are expressed as a percentage of the total fluorescence and represent the mean±SEM from two independent experiments carried out in triplicate.
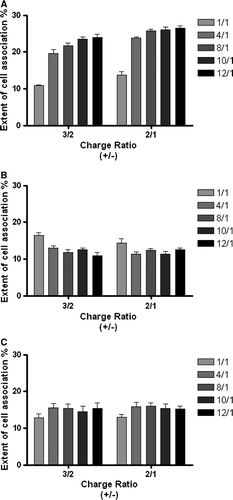
Figure 9. Representative images obtained from fluorescence microscopy for cell association of PEI-complexes (2.0 KDa PEI) prepared by the ethanol injection method from DOTAP:Chol (DC) liposomes associated or not with transferrin, or DOTAP:CHEMS:DOPE:Chol (CatpH-det) liposomes associated with transferrin, at the 3/2 lipid/DNA (+/-) charge ratio and at the indicated N/P ratios (8/1, 10/1 and 12/1. Liposomes were labeled by incorporating Rh-PE into the lipid bilayer, at a concentration of 5 mol% of total lipid and PEI-complexes were visualized after 4 h incubation with HeLa cells under an epifluorescence microscope (Zeiss Axioscope). This figure is reproduced in colour in Molecular Membrane Biology online.
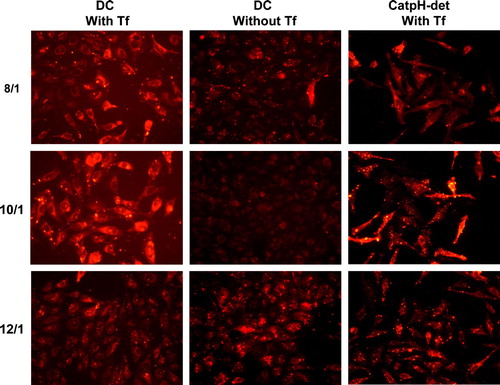
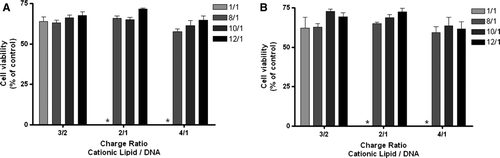
Figure 10. Effect of the lipid/DNA (+/-) charge ratio and N/P ratio PEI-Tf-complexes (2.0 KDa PEI) on the viability of HeLa cells. PEI-Tf-complexes were obtained from DOTAP:Chol (DC) liposomes prepared by the ethanol injection method at lipid/DNA (+/-) charge ratios and N/P ratios that exhibited the highest levels of transfection. Cell viability was measured by the Alamar blue assay at (A) 24 h and (B) 48 h after transfection, as described in Materials and methods and was expressed as the percentage of the untreated cells. The results correspond to the mean±SEM obtained from triplicates, and are representative of, at least, two independent experiments.