Figures & data
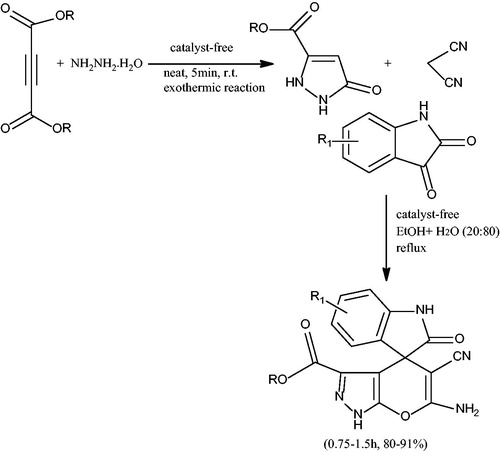
Scheme 2. Synthesis of pyrano[2,3-c]pyrazole derivatives under solvent and catalyst-free conditions.
![Scheme 2. Synthesis of pyrano[2,3-c]pyrazole derivatives under solvent and catalyst-free conditions.](/cms/asset/e819d19f-318b-43a8-8ed6-b8f1df908d95/gpol_a_1584576_c0002_b.jpg)
Scheme 3. Preparation of pyrano[2,3-c]pyrazoles in aqueous ethanol medium under catalyst-free conditions.
![Scheme 3. Preparation of pyrano[2,3-c]pyrazoles in aqueous ethanol medium under catalyst-free conditions.](/cms/asset/c2856d14-680f-4568-b7e5-481d675f98dd/gpol_a_1584576_c0003_b.jpg)
Scheme 4. Synthesis of spiro-pyrano[2,3-c]pyrazoles in aqueous ethanol medium under catalyst-free conditions.
![Scheme 4. Synthesis of spiro-pyrano[2,3-c]pyrazoles in aqueous ethanol medium under catalyst-free conditions.](/cms/asset/d9dff35d-39e1-4c06-9226-8319fb2a3df2/gpol_a_1584576_c0004_b.jpg)
Scheme 5. Four-component catalyst-free synthesis of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates in water.
![Scheme 5. Four-component catalyst-free synthesis of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates in water.](/cms/asset/809cbe7b-03f5-498e-88ae-7900c5c8027b/gpol_a_1584576_c0005_b.jpg)
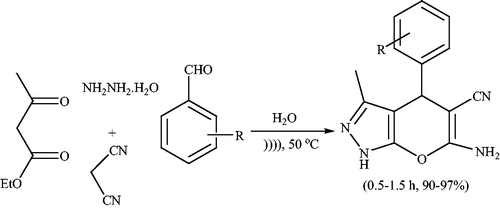
Scheme 7. Catalyst-free synthesis of 6-amino-4-aryl-3-methyl-2,4-dihydropyrano[2,3-c]pyrazolecarbonitriles in boiling wather.
![Scheme 7. Catalyst-free synthesis of 6-amino-4-aryl-3-methyl-2,4-dihydropyrano[2,3-c]pyrazolecarbonitriles in boiling wather.](/cms/asset/302cc78d-eea6-4997-ba70-1d380894e662/gpol_a_1584576_c0007_b.jpg)
Scheme 9. Catalyst-free synthesis of spiro-pyrano[2,3-c]pyrazole derivatives in water/ethanol at 60 °C.
![Scheme 9. Catalyst-free synthesis of spiro-pyrano[2,3-c]pyrazole derivatives in water/ethanol at 60 °C.](/cms/asset/b4571204-a9e9-407d-99b5-f0fe7516c0bd/gpol_a_1584576_c0009_b.jpg)
Scheme 10. Catalyst-free synthesis of 6-amino-4-aryl-3-(trifluoromethyl)-1,4-dihydro-1-phenylpyrano[2,3-c]pyrazole-5-carbonitriles in aqueous media.
![Scheme 10. Catalyst-free synthesis of 6-amino-4-aryl-3-(trifluoromethyl)-1,4-dihydro-1-phenylpyrano[2,3-c]pyrazole-5-carbonitriles in aqueous media.](/cms/asset/4bd5d0b1-a9c3-4062-b4ec-6e1612cb3d14/gpol_a_1584576_c0010_b.jpg)
Scheme 13. Synthesis of the pyrano[2,3-c]pyrazole and pyrano[4',3':5,6]pyrazolo [2,3-d]pyrimidine derivatives using magnetized water.
![Scheme 13. Synthesis of the pyrano[2,3-c]pyrazole and pyrano[4',3':5,6]pyrazolo [2,3-d]pyrimidine derivatives using magnetized water.](/cms/asset/a1f9d2ef-09fb-457f-9356-c751fb0ff3d2/gpol_a_1584576_c0013_b.jpg)
Scheme 16. Synthesis 6-amino-4-aryl-5-cyano-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates by a four-component reaction in the presence of acetic acid.
![Scheme 16. Synthesis 6-amino-4-aryl-5-cyano-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates by a four-component reaction in the presence of acetic acid.](/cms/asset/a725138a-b622-491c-bc1d-58e5f0bae631/gpol_a_1584576_c0016_b.jpg)
Scheme 17. Preparation of pyrido[2,3-c]pyrazoles using HPA supported onto the silica coated NiFe2O4 MNPs (NFS-PRS).
![Scheme 17. Preparation of pyrido[2,3-c]pyrazoles using HPA supported onto the silica coated NiFe2O4 MNPs (NFS-PRS).](/cms/asset/14d77bca-e828-48c0-b7d1-e9ed0b66d3eb/gpol_a_1584576_c0017_b.jpg)
Scheme 18. The use of 1-(carboxymethyl)pyridiniumiodide {[cmpy]I} for the green synthesis of 6-amino-4-(4-methoxyphenyl)-5-cyano-3-methyl-1-phenyl-1,4 dihydropyrano[2,3-c]pyrazoles.
![Scheme 18. The use of 1-(carboxymethyl)pyridiniumiodide {[cmpy]I} for the green synthesis of 6-amino-4-(4-methoxyphenyl)-5-cyano-3-methyl-1-phenyl-1,4 dihydropyrano[2,3-c]pyrazoles.](/cms/asset/43880973-8856-4279-bdd3-a1ec5d069b80/gpol_a_1584576_c0018_b.jpg)
Scheme 22. Synthesis of 4,3'-spiro[(6-amino-5-R-3-methyl-2,4-dihydropyrano[2,3-c]-pyrazolo)-2'-oxindoles] using triethanolamine.
![Scheme 22. Synthesis of 4,3'-spiro[(6-amino-5-R-3-methyl-2,4-dihydropyrano[2,3-c]-pyrazolo)-2'-oxindoles] using triethanolamine.](/cms/asset/db6e5b72-c6ac-41cd-acd0-10c89584ae64/gpol_a_1584576_c0022_b.jpg)
Scheme 23. Preparation of 6-amino-5-cyano-3-methyl-4-aryl/heteroaryl-2H,4H-dihydropyrano[2,3-c]pyrazoles in the presence of piperidine.
![Scheme 23. Preparation of 6-amino-5-cyano-3-methyl-4-aryl/heteroaryl-2H,4H-dihydropyrano[2,3-c]pyrazoles in the presence of piperidine.](/cms/asset/ca70e44c-d3cc-4c16-9160-ad48366dd2dd/gpol_a_1584576_c0023_b.jpg)
Scheme 25. Four-component synthesis of 6-amino-2H,4Hpyrano[2,3-c]pyrazol-5-carbonitriles using aromatic aldehyde.
![Scheme 25. Four-component synthesis of 6-amino-2H,4Hpyrano[2,3-c]pyrazol-5-carbonitriles using aromatic aldehyde.](/cms/asset/34b9efcf-7e22-4ed7-b72c-be026cb4765c/gpol_a_1584576_c0025_b.jpg)
Scheme 29. Piperidine catalyzed synthesis of spiro[indoline-3,4'-pyrano[2,3-c]pyrazole]-5'-carbonitriles.
![Scheme 29. Piperidine catalyzed synthesis of spiro[indoline-3,4'-pyrano[2,3-c]pyrazole]-5'-carbonitriles.](/cms/asset/237c2d0e-9960-4f26-ab7e-60a86dbcd3c7/gpol_a_1584576_c0029_b.jpg)
Scheme 30. Piperidine catalyzed synthesis of spiro[acenaphthylene-1,4'-pyrano[2,3-c]pyrazole]-5'-carbonitrile.
![Scheme 30. Piperidine catalyzed synthesis of spiro[acenaphthylene-1,4'-pyrano[2,3-c]pyrazole]-5'-carbonitrile.](/cms/asset/f543e933-204a-43fc-af9f-46f722e62176/gpol_a_1584576_c0030_b.jpg)
Scheme 31. Synthesis of 3-methyl-4-aryl-4,5-dihydro-1H-pyrano[2,3-c]pyrazol-6-ones in the presence of Ba(OH)2.
![Scheme 31. Synthesis of 3-methyl-4-aryl-4,5-dihydro-1H-pyrano[2,3-c]pyrazol-6-ones in the presence of Ba(OH)2.](/cms/asset/b6b19697-1ae9-44f3-8c5f-0f805d23ff8f/gpol_a_1584576_c0031_b.jpg)
Scheme 32. Preparation of 6-amino-3-methyl-4-aryl-/1-phenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles using cetyltrimethylammonium chloride (CTACl).
![Scheme 32. Preparation of 6-amino-3-methyl-4-aryl-/1-phenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles using cetyltrimethylammonium chloride (CTACl).](/cms/asset/986d9fda-9a42-4a3b-a48c-3eabcda49731/gpol_a_1584576_c0032_b.jpg)
Scheme 33. Base catalyzed synthesis of spiro[indoline-3,4'-pyrano[2,3-c]pyrazole]-3'-carboxylate derivatives.
![Scheme 33. Base catalyzed synthesis of spiro[indoline-3,4'-pyrano[2,3-c]pyrazole]-3'-carboxylate derivatives.](/cms/asset/0ab84216-d672-4081-bcdb-56513556a600/gpol_a_1584576_c0033_b.jpg)
Scheme 35. (E)-N-Methyl-1-(methylthio)-2-nitroethenamine (NMSM) as an ambiphilic synthon for the synthesis of pyrano[2,3-c]pyrazoles.
![Scheme 35. (E)-N-Methyl-1-(methylthio)-2-nitroethenamine (NMSM) as an ambiphilic synthon for the synthesis of pyrano[2,3-c]pyrazoles.](/cms/asset/29bf524d-cfc3-41f7-963b-71401ae36980/gpol_a_1584576_c0035_b.jpg)
Scheme 36. Preparation of 6-amino-5-cyano-2′-oxo-5′-phenyl-1′,2′-dihydro-1H-spiro[pyrano[2,3-c]pyrazole-4,3′-pyrroles] in the presence of NEt3.
![Scheme 36. Preparation of 6-amino-5-cyano-2′-oxo-5′-phenyl-1′,2′-dihydro-1H-spiro[pyrano[2,3-c]pyrazole-4,3′-pyrroles] in the presence of NEt3.](/cms/asset/673ea8a5-e269-47bb-bad3-a95c500d752b/gpol_a_1584576_c0036_b.jpg)
Scheme 37. Synthesis of coumarin based pyrano[2,3-c]pyrazole derivatives using DMAP (4-dimethylaminopyridinein).
![Scheme 37. Synthesis of coumarin based pyrano[2,3-c]pyrazole derivatives using DMAP (4-dimethylaminopyridinein).](/cms/asset/19564201-d469-49ee-b276-eea714b4a237/gpol_a_1584576_c0037_b.jpg)
Scheme 38. Diethyl oxalacetate sodium salt as a reagent for the synthesis of spiro[indoline-3,4'-pyrano[2,3-c]pyrazoles].
![Scheme 38. Diethyl oxalacetate sodium salt as a reagent for the synthesis of spiro[indoline-3,4'-pyrano[2,3-c]pyrazoles].](/cms/asset/29f55484-aa94-43bd-8201-e3ea43d223d1/gpol_a_1584576_c0038_b.jpg)
Scheme 39. A plausible mechanism for the preparation of spiro[indoline-3,4'-pyrano[2,3-c]pyrazoles].
![Scheme 39. A plausible mechanism for the preparation of spiro[indoline-3,4'-pyrano[2,3-c]pyrazoles].](/cms/asset/62dd66f4-00f6-4f0d-a8e5-54dcc9416c1f/gpol_a_1584576_c0039_b.jpg)
Scheme 40. Application of N,N-Diisopropylethylamine (DIPEA) for the synthesis of 1,4-dihydropyrano[2,3-c]pyrazol-6-amines.
![Scheme 40. Application of N,N-Diisopropylethylamine (DIPEA) for the synthesis of 1,4-dihydropyrano[2,3-c]pyrazol-6-amines.](/cms/asset/57c0fd39-b747-424b-bd39-8f9e6f872d13/gpol_a_1584576_c0040_b.jpg)
Scheme 47. Synthesis of 6'-amino-1-(4-(6'-amino-5'-cyano-3'-methyl-2-oxo-1'H-spiro[indoline-3,4'-pyrano[2,3-c]pyrazol]-1-yl)butyl)-3'-methyl-2-oxo-1'H-spiro[indoline-3,4'-pyrano[2,3-c]pyrazole]-5'-carbonitrile in the presence of β-CD.
![Scheme 47. Synthesis of 6'-amino-1-(4-(6'-amino-5'-cyano-3'-methyl-2-oxo-1'H-spiro[indoline-3,4'-pyrano[2,3-c]pyrazol]-1-yl)butyl)-3'-methyl-2-oxo-1'H-spiro[indoline-3,4'-pyrano[2,3-c]pyrazole]-5'-carbonitrile in the presence of β-CD.](/cms/asset/7958e0a4-4a5c-491b-b5f4-e787491f0e5f/gpol_a_1584576_c0047_b.jpg)
Scheme 48. Per-6-amino-β-cyclodextrin (per-6-ABCD) as catalyst for the synthesis of dihydropyrano[2,3-c]pyrazole derivatives.
![Scheme 48. Per-6-amino-β-cyclodextrin (per-6-ABCD) as catalyst for the synthesis of dihydropyrano[2,3-c]pyrazole derivatives.](/cms/asset/0b6732e1-64eb-4ca3-bf1c-2bb5d2eeb8c5/gpol_a_1584576_c0048_b.jpg)
Scheme 41. Synthesis of 1,4-dihydropyrano[2,3-c]pyrazoles using N,2-dibromo-6-chloro-3,4-dihydro-2H-benzo[e][1,2,4] thiadiazine-7-sulfonamide-1,1-dioxide (DCDBTSD).
![Scheme 41. Synthesis of 1,4-dihydropyrano[2,3-c]pyrazoles using N,2-dibromo-6-chloro-3,4-dihydro-2H-benzo[e][1,2,4] thiadiazine-7-sulfonamide-1,1-dioxide (DCDBTSD).](/cms/asset/66fddde5-c10b-423b-8f87-385b3986a078/gpol_a_1584576_c0041_b.jpg)
Scheme 51. Enantioselective synthesis of 6-amino-5 cyanodihydropyrano[2,3-c]pyrazoles using cupreine (37).
![Scheme 51. Enantioselective synthesis of 6-amino-5 cyanodihydropyrano[2,3-c]pyrazoles using cupreine (37).](/cms/asset/b24c461a-2c21-46cf-a4ef-80eee3aef472/gpol_a_1584576_c0051_b.jpg)
Scheme 62. Preparation of spiro[acenaphthylene-1,4'-pyrano[2,3-c]pyrazole] meglumine using meglumine.
![Scheme 62. Preparation of spiro[acenaphthylene-1,4'-pyrano[2,3-c]pyrazole] meglumine using meglumine.](/cms/asset/246c0018-5815-4742-ab17-b3dcc340c714/gpol_a_1584576_c0062_b.jpg)
Scheme 67. Synthesis of spiro[indoline-3,4'-pyrano[2,3-c]pyrazole] derivatives in the presence of TBBDA or PBBS.
![Scheme 67. Synthesis of spiro[indoline-3,4'-pyrano[2,3-c]pyrazole] derivatives in the presence of TBBDA or PBBS.](/cms/asset/bb12a941-3fed-4308-b4fd-9fd288d1d2aa/gpol_a_1584576_c0067_b.jpg)
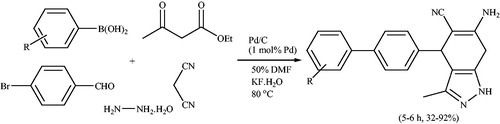
Scheme 70. Five-component reaction for the synthesis a series of biaryl substituted pyranopyrazoles using Pd/C.
Scheme 71. Four-component synthesis of pyranopyrazoles using a mixed-ligand Ni(II) complex [Ni(L)(mimi)].
![Scheme 71. Four-component synthesis of pyranopyrazoles using a mixed-ligand Ni(II) complex [Ni(L)(mimi)].](/cms/asset/f20ccbc3-361a-4f56-b7dd-5c67a6c0a7fe/gpol_a_1584576_c0071_b.jpg)
Scheme 73. Synthesis of 6-amino-4-alkyl/aryl-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-carbonitriles using Amberlyst A21.
![Scheme 73. Synthesis of 6-amino-4-alkyl/aryl-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-carbonitriles using Amberlyst A21.](/cms/asset/9aca63eb-e70b-4fed-85c7-6eeba55ae79a/gpol_a_1584576_c0073_b.jpg)
Scheme 75. Utilization of PEG-400 for the synthesis of spiro[indoline-3,4'-pyrano[2,3-c]pyrazole]-3'-carboxylates.
![Scheme 75. Utilization of PEG-400 for the synthesis of spiro[indoline-3,4'-pyrano[2,3-c]pyrazole]-3'-carboxylates.](/cms/asset/9757774a-2e91-4988-b47b-21ad2b56480d/gpol_a_1584576_c0075_b.jpg)
Scheme 76. The use of PEG-400 for the synthesis of trifluoromethylated spiro[indole-3,4'-pyrano[2,3-c]pyrazole] derivatives.
![Scheme 76. The use of PEG-400 for the synthesis of trifluoromethylated spiro[indole-3,4'-pyrano[2,3-c]pyrazole] derivatives.](/cms/asset/5eef71cd-90dc-4a28-a114-360d858e9a21/gpol_a_1584576_c0076_b.jpg)
Scheme 79. Synthesis of 6-amino-4-(4-methoxyphenyl)-5-cyano-3-methyl-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazoles using [Dsim]AlCl4.
![Scheme 79. Synthesis of 6-amino-4-(4-methoxyphenyl)-5-cyano-3-methyl-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazoles using [Dsim]AlCl4.](/cms/asset/754b441c-cdc8-49da-b253-b625e12046eb/gpol_a_1584576_c0079_b.jpg)
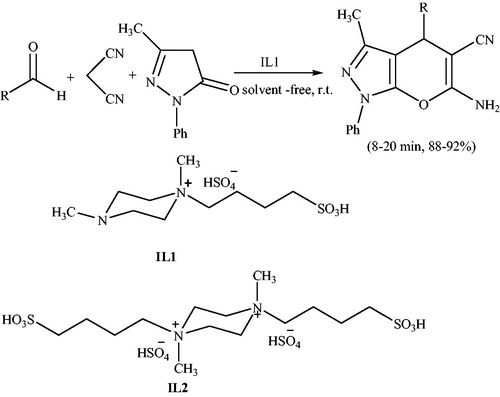
Scheme 81. Synthesis of pyranopyrazoles using 1,4-dimethyl-1-(4-sulphobutyl)piperazinium hydrogen sulfate.
Scheme 85. Methyl 4-(aryl)-3-methyl-6-oxo-1,4,5,6-tetrahydropyrano[2,3-c] pyrazole-5- carboxylates using [DMBSI]HSO4.
![Scheme 85. Methyl 4-(aryl)-3-methyl-6-oxo-1,4,5,6-tetrahydropyrano[2,3-c] pyrazole-5- carboxylates using [DMBSI]HSO4.](/cms/asset/167a453d-871d-4d91-a2e7-f29858dde33f/gpol_a_1584576_c0085_b.jpg)
Scheme 87. Supported ionic liquids catalyzed synthesis of 3-methyl-4-aryl-4,5-dihydro-1H-pyrano[2,3-c]pyrazol-6-ones.
![Scheme 87. Supported ionic liquids catalyzed synthesis of 3-methyl-4-aryl-4,5-dihydro-1H-pyrano[2,3-c]pyrazol-6-ones.](/cms/asset/116de054-5ecc-4f36-a04c-aced156d50c0/gpol_a_1584576_c0087_b.jpg)
Scheme 89. Preparation of 6-amino-2H,4H-pyrano[2,3-c]pyrazole-5-carbonitriles using DES (choline chloride:urea).
![Scheme 89. Preparation of 6-amino-2H,4H-pyrano[2,3-c]pyrazole-5-carbonitriles using DES (choline chloride:urea).](/cms/asset/de49e9cd-1e25-4457-b07a-e51aacc3f254/gpol_a_1584576_c0089_b.jpg)
Scheme 91. Synthesis of 6-amino-4-aryl-2,4-dihydro-3-phenyl pyrano [2,3-c]pyrazole-5-carbonitrile derivatives using choline chloride based thiourea.
![Scheme 91. Synthesis of 6-amino-4-aryl-2,4-dihydro-3-phenyl pyrano [2,3-c]pyrazole-5-carbonitrile derivatives using choline chloride based thiourea.](/cms/asset/a62e6c4b-c963-42e2-bd8b-9760e03b3067/gpol_a_1584576_c0091_b.jpg)
Scheme 92. Synthesis of 6-amino-3-alkyl-4-aryl-5-cyano-1,4-dihydropyrano[2,3-c]pyrazoles in the presence of magnesium oxide.
![Scheme 92. Synthesis of 6-amino-3-alkyl-4-aryl-5-cyano-1,4-dihydropyrano[2,3-c]pyrazoles in the presence of magnesium oxide.](/cms/asset/8624b593-24e2-4a15-a7ca-cc4577eb3951/gpol_a_1584576_c0092_b.jpg)
Scheme 94. The use of CuFe2O4 magnetic nanoparticles for the synthesis of 3-methyl-1,4-dihydropyrano[2,3-c]pyrazoles.
![Scheme 94. The use of CuFe2O4 magnetic nanoparticles for the synthesis of 3-methyl-1,4-dihydropyrano[2,3-c]pyrazoles.](/cms/asset/c8e15ac9-f901-44cc-8ac1-bf134ca2fbd6/gpol_a_1584576_c0094_b.jpg)
Scheme 95. Preparation of dihydropyrano[2,3-c]pyrazole-3-carboxylate derivatives in the presence of CuFe2O4 magnetic nanoparticles.
![Scheme 95. Preparation of dihydropyrano[2,3-c]pyrazole-3-carboxylate derivatives in the presence of CuFe2O4 magnetic nanoparticles.](/cms/asset/66121ba6-c376-4a5e-a83f-ade22630ffb4/gpol_a_1584576_c0095_b.jpg)
Scheme 97. The use of nano titanium dioxide for preparation of 6-amino-4-aryl-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles.
![Scheme 97. The use of nano titanium dioxide for preparation of 6-amino-4-aryl-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles.](/cms/asset/09670c99-0801-4bb9-95a6-495680c581e0/gpol_a_1584576_c0097_b.jpg)
Scheme 98. Application of ZrO2 nanoparticles as catalyst in the synthesis of pyrano[2,3-c]pyrazoles.
![Scheme 98. Application of ZrO2 nanoparticles as catalyst in the synthesis of pyrano[2,3-c]pyrazoles.](/cms/asset/9f072f85-596a-4783-8d36-81a20a4237e0/gpol_a_1584576_c0098_b.jpg)
Scheme 102. The use of BF3/MNPs nanoparticles for preparation of 1,4-dihydropyrano[2,3-c]pyrazole derivatives.
![Scheme 102. The use of BF3/MNPs nanoparticles for preparation of 1,4-dihydropyrano[2,3-c]pyrazole derivatives.](/cms/asset/22a184ae-71b4-42ea-983d-f5bcf4f14ac1/gpol_a_1584576_c0102_b.jpg)
Scheme 103. Synthesis of pyran[2,3-c]pyrazoles in the presence of Fe3O4@SiO2 core-shell nanocatalyst.
![Scheme 103. Synthesis of pyran[2,3-c]pyrazoles in the presence of Fe3O4@SiO2 core-shell nanocatalyst.](/cms/asset/27204c74-0239-4c87-bd07-dafd5bde1e2a/gpol_a_1584576_c0103_b.jpg)
Scheme 105. Synthesis of 3-methyl-4-phenyl-1,4-dihydropyrazolo[4',3':5,6]pyrano[2,3-d]pyrimidine-5,7(6H,8H)-dione derivatives.
![Scheme 105. Synthesis of 3-methyl-4-phenyl-1,4-dihydropyrazolo[4',3':5,6]pyrano[2,3-d]pyrimidine-5,7(6H,8H)-dione derivatives.](/cms/asset/0dd13a05-413b-4fe3-9354-1d9f5fa56861/gpol_a_1584576_c0105_b.jpg)
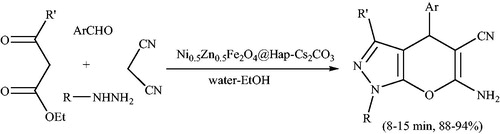
Scheme 110. The use of Ni0.5Zn0.5Fe2O4 (Ni0.5Zn0.5Fe2O4–PPA) for preparation of 5-cyano-1,4-dihydropyrano[2,3-c]pyrazoles.
![Scheme 110. The use of Ni0.5Zn0.5Fe2O4 (Ni0.5Zn0.5Fe2O4–PPA) for preparation of 5-cyano-1,4-dihydropyrano[2,3-c]pyrazoles.](/cms/asset/b86bdf8d-f431-4aa2-9fcf-44f9637c5fbd/gpol_a_1584576_c0110_b.jpg)
Scheme 116. synthesis of dihydropyrano[2,3-c]pyrazole derivatives in the presence of Fe3O4@HNTs-PEI nano-catalyst.
![Scheme 116. synthesis of dihydropyrano[2,3-c]pyrazole derivatives in the presence of Fe3O4@HNTs-PEI nano-catalyst.](/cms/asset/9ef02d89-414f-47cb-8adf-422b79039178/gpol_a_1584576_c0116_b.jpg)
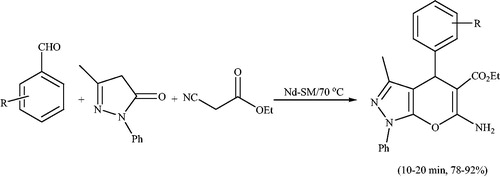
Scheme 118. Synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives using β-CD/EP polymer.
![Scheme 118. Synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives using β-CD/EP polymer.](/cms/asset/ea7a4e12-54ef-48bb-996c-12920c479337/gpol_a_1584576_c0118_b.jpg)
Scheme 122. Presented mechanism for the electrosynthesis of spirocyclic [indole-3,4'-pyrano[2,3-c]pyrazoles].
![Scheme 122. Presented mechanism for the electrosynthesis of spirocyclic [indole-3,4'-pyrano[2,3-c]pyrazoles].](/cms/asset/e7b1bf7e-ca9b-4e4f-9651-938b2e824fe2/gpol_a_1584576_c0122_b.jpg)
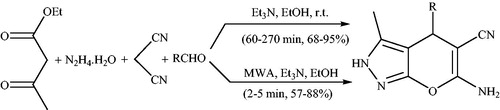
Scheme 123. Preparation of pyranopyrazoles by the traditional heating techniques and microwave-assisted reaction.
Scheme 129. Synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives in the presence of piperidine under ultrasonic irradiations.
![Scheme 129. Synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives in the presence of piperidine under ultrasonic irradiations.](/cms/asset/2b80df28-2caf-4fe0-9505-917602848303/gpol_a_1584576_c0129_b.jpg)
Scheme 130. Preparation of dihydropyrano[2,3-c]pyrazole derivatives in the presence of CAN and under ultrasonic irradiation.
![Scheme 130. Preparation of dihydropyrano[2,3-c]pyrazole derivatives in the presence of CAN and under ultrasonic irradiation.](/cms/asset/3231d490-158a-442a-b68e-435dbcf21ce9/gpol_a_1584576_c0130_b.jpg)

![Scheme 1. Catalyst-free synthesis of a series of pyrano[2,3-c]pyrazoles.](/cms/asset/28595728-c8be-4f86-aa77-b851a2b0f275/gpol_a_1584576_c0001_b.jpg)
![Scheme 8. Catalyst-free synthesis of pyrano[2,3-c]pyrazole derivatives in water/ethanol at 60 °C.](/cms/asset/0f240470-aca3-4e9d-89db-70d6a242ad5a/gpol_a_1584576_c0008_b.jpg)
![Scheme 11. Catalyst-free synthesis of dihydropyrano[2,3-c]pyrazoles using ball milling technique](/cms/asset/2f85487f-b0e3-493f-b027-e43bdec7b06b/gpol_a_1584576_c0011_b.jpg)
![Scheme 12. Catalyst-free synthesis of pyrazolo[4',3':5,6]Pyrano[2,3-d]Pyrimidines.](/cms/asset/41210fbd-c255-404e-8630-a85e808c6499/gpol_a_1584576_c0012_b.jpg)
![Scheme 14. Solvent-free synthesis of thioether containing dihydropyrano[2,3-c]pyrazoles.](/cms/asset/48afcc7f-2e8f-4223-a951-d04642ddfb09/gpol_a_1584576_c0014_b.jpg)
![Scheme 15. Synthesis of pyrano[2,3-c]pyrazoles in the presence of silicotungstic acid.](/cms/asset/ff848827-1038-4267-868d-1960b8ee2601/gpol_a_1584576_c0015_b.jpg)
![Scheme 19. Synthesis of pyrano[2,3-c]pyrazoles using montmorillonite-K10.](/cms/asset/b59ee27e-961f-423e-8959-d41526edf8c1/gpol_a_1584576_c0019_b.jpg)
![Scheme 21. The use of aqueous solution of boric acid for the synthesis of pyrano[2,3-c]pyrazoles.](/cms/asset/9a5b4ed8-27bf-4d20-8263-b7c1a954fa50/gpol_a_1584576_c0021_b.jpg)
![Scheme 24. Synthesis of dihydropyrano[2,3-c]pyrazoles in EtOH at room temperature.](/cms/asset/a597847b-331c-4c5d-bd1e-afdc0bf0f944/gpol_a_1584576_c0024_b.jpg)
![Scheme 27. Synthesis of spiro-conjugated 6-amino-2H,4H-pyrano[2,3-c]pyrazol-5-carbonitriles.](/cms/asset/0554ff83-f7cf-4c4a-813f-704abb93349e/gpol_a_1584576_c0027_b.jpg)
![Scheme 26. Four-component synthesis of pyrano[2,3-c]pyrazole using saturated heterocyclic ketones.](/cms/asset/cf775164-dea5-453d-9c0f-e5b797f8de3c/gpol_a_1584576_c0026_b.jpg)
![Scheme 34. Synthesis of pyrano[2,3-c]pyrazoles in the presence of sodium benzoate.](/cms/asset/c56518db-82fb-446c-bc44-c299ebb81d17/gpol_a_1584576_c0034_b.jpg)
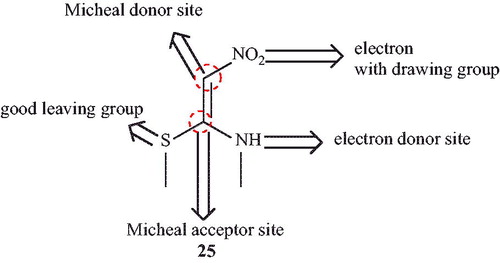
![Scheme 42. Synthesis of spiro-pyrano[2,3-c]pyrazoles in the presence of DCDBTSD.](/cms/asset/3eef58fe-f4f7-4306-a77e-58e0fd9023d4/gpol_a_1584576_c0042_b.jpg)
![Scheme 43. NMO and Ag2O catalyzed synthesis of pyrano[2,3-c]pyrazoles.](/cms/asset/89e530d8-615a-4625-8a73-ee52e927506e/gpol_a_1584576_c0043_b.jpg)
![Scheme 46. Synthesis of spiro-pyrano[2,3-c]pyrazoles using β-CD.](/cms/asset/ab063719-5e12-4ace-a855-c5c11a3cef6d/gpol_a_1584576_c0046_b.jpg)
![Scheme 49. Synthesis of pyrano[2,3-c]pyrazoles using imidazole.](/cms/asset/c265c8a6-43d7-4057-8ad6-bf19c5e0a8a1/gpol_a_1584576_c0049_b.jpg)
![Scheme 50. Urea catalyzed synthesis of 1,4-dihydropyrano[2,3-c]pyrazoles.](/cms/asset/6e78447b-77ac-49fd-9c98-1de9dff540fe/gpol_a_1584576_c0050_b.jpg)
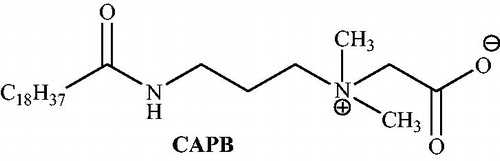
![Scheme 52. Synthesis of dihydropyrano[2,3-c]pyrazoles using cocamidopropyl betaine (CAPB).](/cms/asset/6883d057-3d11-4a4e-b367-0a6bbc2f3ba8/gpol_a_1584576_c0052_b.jpg)
![Scheme 45. Synthesis of pyrano[2,3-c]pyrazoles using β-CD.](/cms/asset/ad879c62-ab27-446d-94c9-0f5476289e12/gpol_a_1584576_c0045_b.jpg)
![Scheme 53. Isonicotinic acid catalyzed synthesis of 1,4-dihydropyrano[2,3-c]pyrazoles.](/cms/asset/c189e796-c7a1-430c-a975-76ef1a726b5b/gpol_a_1584576_c0053_b.jpg)
![Scheme 54. Synthesis of pyrano[2,3-c]pyrazole using bleaching earth clay.](/cms/asset/0e927293-84ae-462f-918a-bbc6a000bce9/gpol_a_1584576_c0054_b.jpg)
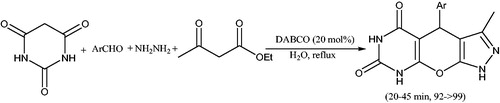
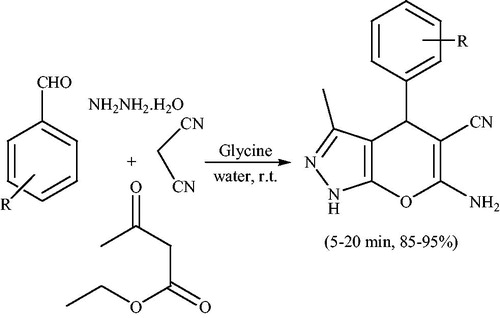
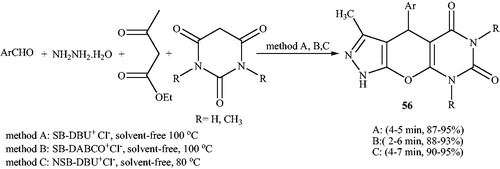
![Scheme 56. Synthesis of dihydropyrano[2,3-c]pyrazole derivatives using DABCO.](/cms/asset/ad37069c-dfd1-485a-b752-eb7f95935a15/gpol_a_1584576_c0056_b.jpg)
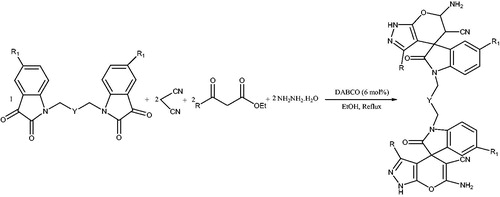
![Scheme 58. Synthesis of 1,4-dihydropyrano[2,3-c]pyrazoles using organocatalysts.](/cms/asset/fbe3d666-38f4-45ad-afff-2112bab00e54/gpol_a_1584576_c0058_b.jpg)
![Scheme 59. Grinding protocol for the synthesis of dihydropyrano[2,3-c]pyrazole using L-proline.](/cms/asset/9d109ca0-078b-47df-bb71-f6ccc89fff61/gpol_a_1584576_c0059_b.jpg)
![Scheme 61. Synthesis of spiro[indoline-3,4' pyrano[2,3-c]pyrazole] derivatives using meglumine.](/cms/asset/00b431f4-86d5-4e32-b84e-531caa9d67cf/gpol_a_1584576_c0061_b.jpg)
![Scheme 63. The use of maltose to synthesis of 1,4-dihydropyrano[2,3-c]pyrazoles.](/cms/asset/2fb4c289-9912-4729-8ea1-b75bb228a9a5/gpol_a_1584576_c0063_b.jpg)
![Scheme 64. Application of sucrose to synthesis of 1,4-dihydropyrano[2,3-c]pyrazoles.](/cms/asset/b0b4dae5-d081-45d2-81bf-2e67217d9c6d/gpol_a_1584576_c0064_b.jpg)
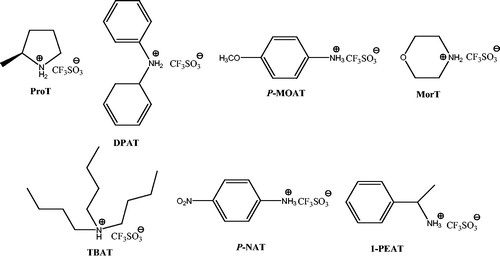
![Scheme 65. The use of morpholine triflate (MorT) to prepare dihydropyrano[2,3-c]pyrazoles.](/cms/asset/337b19df-6d3b-4293-a48a-b7adb7abd139/gpol_a_1584576_c0065_b.jpg)
![Scheme 66. Synthesis of pyrano[2,3-c]pyrazole derivatives using Bovine Serum Albumin (BSA).](/cms/asset/d3956034-a209-43f8-8263-b8c980bc5aad/gpol_a_1584576_c0066_b.jpg)
![Scheme 60. Synthesis of a series of dihydroprano[2,3-c]pyrazoles in the presence of meglumine.](/cms/asset/f333ea1c-2b6a-4454-8829-56a26f9ba1c1/gpol_a_1584576_c0060_b.jpg)
![Scheme 68. Synthesis of pyrano[2,3-c]pyrazoles in the presence of Ph3CCl.](/cms/asset/be687475-9577-48a5-9919-1fa58a77ac17/gpol_a_1584576_c0068_b.jpg)
![Scheme 69. Dihydropyrano[2,3-c]pyrazoles and spiro-pyranopyrazoles in the presence of aspirin.](/cms/asset/9bf3f1c3-38f2-42af-bbfa-7e9ba478fcd0/gpol_a_1584576_c0069_b.jpg)
![Scheme 72. The use of Fe-CaOx for the synthesis of 2,4-dihydropyrano[2,3-c]pyrazoles.](/cms/asset/60789324-4467-4999-a77e-3fc4e8b5084d/gpol_a_1584576_c0072_b.jpg)
![Scheme 74. Synthesis of dihydropyrano[2,3-c]pyrazole derivatives using PS-PTSA.](/cms/asset/d28682bc-296a-4cb9-8bb6-e98770e1972f/gpol_a_1584576_c0074_b.jpg)
![Scheme 77. Preparation of 4H-pyrano[2,3-c]pyrazoles in the presence of L-proline and [Bmim]BF4.](/cms/asset/654a3cb4-28c8-4d60-8acf-d26ddbe63b7f/gpol_a_1584576_c0077_b.jpg)
![Scheme 78. The use of [(CH2)4SO3HMIM][HSO4] for preparation of dihydropyrano[2,3-c]pyrazoles.](/cms/asset/3f0fb810-ce70-48e6-aae0-0a38783feda3/gpol_a_1584576_c0078_b.jpg)
![Scheme 80. The use of [TMG][Ac] for the synthesis of dihydro-1H-pyrano[2,3-c]pyrazol-6-ones.](/cms/asset/7203944d-4d28-4967-80b8-7aa7b5af2c3b/gpol_a_1584576_c0080_b.jpg)
![Scheme 82. The use of [ChCl][ZnCl2]2 or [H-NMP]HSO4 for the synthesis of pyrano[2,3-c]pyrazoles.](/cms/asset/fd2d886b-03fe-4a0e-8425-9356272eb671/gpol_a_1584576_c0082_b.jpg)
![Figure 6. Synthesis of bis-1,4-dihydro-3-methylpyrano[2,3-c]pyrazole-5-carbonitriles.](/cms/asset/d9f19f07-0ebc-4e1e-9208-ef4309968b12/gpol_a_1584576_f0006_b.jpg)
![Scheme 84. Application of [DMBSI]HSO4 to the synthesis of spiro-pyrano[2,3-c]pyrazoles.](/cms/asset/147a2f72-8b1e-4030-928e-2cdf3c298ef1/gpol_a_1584576_c0084_b.jpg)
![Figure 7. Preparation of bis-pyrano[2,3-c]pyrazoles using [DMBSI]HSO4.](/cms/asset/05ede308-9fde-4ee5-8e71-5a5ad7a6b0a3/gpol_a_1584576_f0007_b.jpg)
![Figure 8. Various ionic liquids screened fo the synthesis of pyrano[2,3-c]pyrazole.](/cms/asset/bca78a90-bebd-448b-a8ab-41a207329772/gpol_a_1584576_f0008_b.jpg)
![Scheme 86. Preparation of pyrano[2,3-c]pyrazoles in the presence of [DMDBSI].2HSO4](/cms/asset/8e1cad22-ace7-4efb-9d49-b847e671ad1a/gpol_a_1584576_c0086_b.jpg)
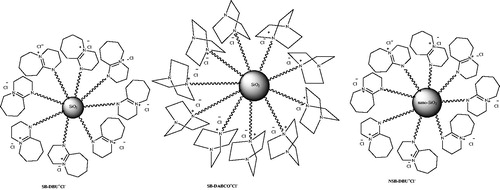
![Scheme 90. Synthesis of dihydropyrano[2,3-c]pyrazole in DES medium.](/cms/asset/c9e3f25a-fd7e-4373-8e1e-23fc61bd0f7f/gpol_a_1584576_c0090_b.jpg)
![Scheme 83. Synthesis of pyrano[2,3-c]pyrazole derivatives in the presence of [DMBSI]HSO4.](/cms/asset/79cca7c7-9452-488c-a13f-5c6d35891d3e/gpol_a_1584576_c0083_b.jpg)
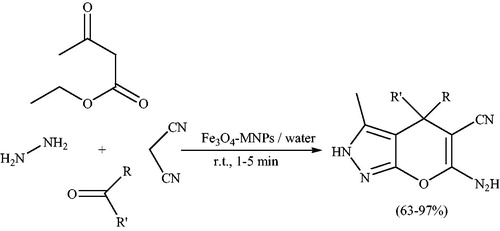
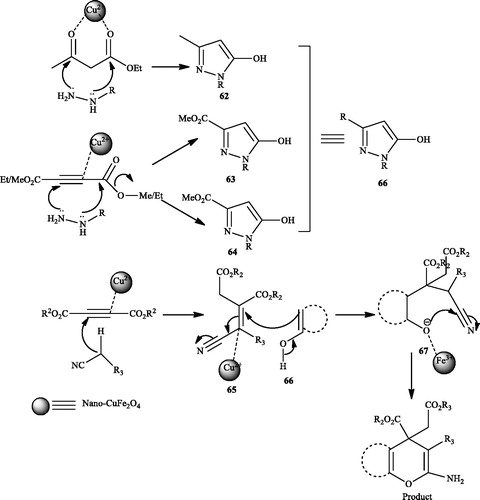
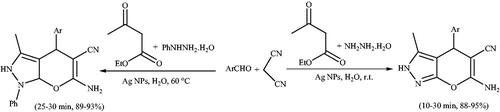
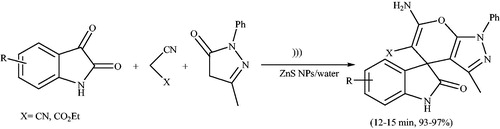
![Scheme 101. The use of ZnS nanoparticles for preparation of 4H-pyrano[2,3-c]pyrazoles by grinding.](/cms/asset/83355f92-dbe7-45d3-9298-d64a428c9260/gpol_a_1584576_c0101_b.jpg)
![Scheme 104. Preparation of 1,4-dihydropyrano[2,3-c]pyrazoles using Fe3-xTixO4@SO3H nanoparticles.](/cms/asset/0f8ea966-7bca-40c6-bb16-018ffb92a341/gpol_a_1584576_c0104_b.jpg)
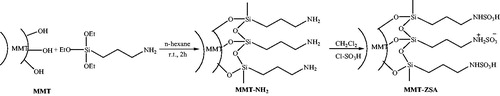
![Scheme 108. Synthesis of dihydropyrano[2,3-c]pyrazole in the presence of Ag/TiO2 nano-thin films.](/cms/asset/d31b94d1-5aba-43e3-be6d-8f42d542d689/gpol_a_1584576_c0108_b.jpg)
![Scheme 109. Synthesis of pyrano[2,3-c:6,5-c']dipyrazol]-2-ones using Ag NPs/rGO composite.](/cms/asset/d439877a-0655-4174-9af2-f05f5b0f7258/gpol_a_1584576_c0109_b.jpg)
![Scheme 111. Synthesis of pyrano[2,3-c]pyrazole in the presence of SiO2 NPs.](/cms/asset/b1453c65-eaa4-4c6b-ab2c-2522c6a1f46c/gpol_a_1584576_c0111_b.jpg)
![Scheme 112. Preparation of nano‐Fe [phenylsalicylaldiminemethylpyranopyrazole]Cl2 catalyst.](/cms/asset/d396a5ec-404e-414c-b6bd-ad75e6c2c4fb/gpol_a_1584576_c0112_b.jpg)
![Scheme 113. Synthesis of pyranopyrazoles by using nano‐[Fe‐PSMP]Cl2.](/cms/asset/b963be7d-4ba1-4bcc-b58f-cff4d8a0e770/gpol_a_1584576_c0113_b.jpg)

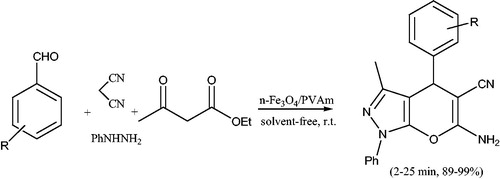
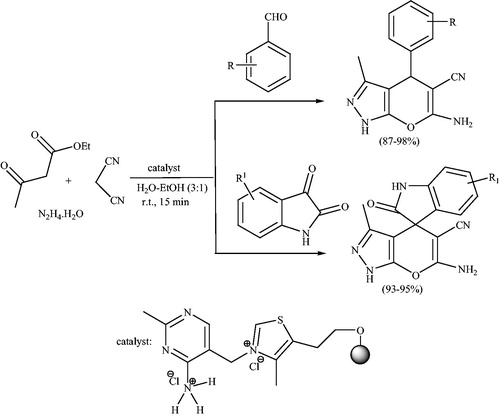
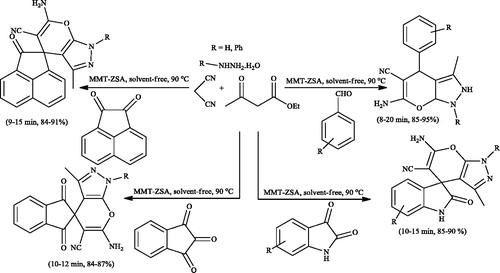
![Scheme 121. Electrosynthesis of spirocyclic [indole-3,4'-pyrano[2,3-c]pyrazole] compounds.](/cms/asset/f48bc8ec-cea3-478d-9165-b468aece7cd8/gpol_a_1584576_c0121_b.jpg)
![Scheme 126. Synthesis of indolylpyrano[2,3-c]pyrazoles under microwave irradiation.](/cms/asset/200a938a-0f2b-494e-8e6c-e01bcfed402a/gpol_a_1584576_c0126_b.jpg)
![Scheme 127. Synthesis of pyrano[2,3-c] pyrazoles using ([bmim][OH] under microwave irradiation.](/cms/asset/46272b96-7448-4a18-98b8-7441333a25b3/gpol_a_1584576_c0127_b.jpg)
![Scheme 131. Preparation of dihydropyrano[2,3-c]pyrazole using molecular sieves (MS 4 Å) under reflux and ultrasonic irradiation.](/cms/asset/039374d1-ed93-4faa-9eaa-9165b96d5554/gpol_a_1584576_c0131_b.jpg)
![Scheme 125. Synthesis of quinolylpyrano[2,3-c]pyrazoles under microwave irradiation.](/cms/asset/53381494-301d-46f5-8790-9b2a70bb469c/gpol_a_1584576_c0125_b.jpg)
![Scheme 133. Synthesis of pyrano[2,3-c]pyrazole-3-carboxylate/pyrano[2,3-c]pyrazole-5-carbonitrile using Mn doped zirconia under ultrasonic irradiation.](/cms/asset/46db3491-a447-4774-9c30-0fa85a2e85bc/gpol_a_1584576_c0133_b.jpg)