Figures & data
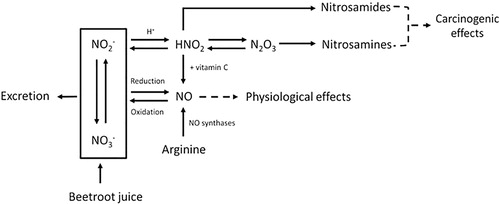
Figure 1. Metabolic pathway of nitrate (NO3–), nitrite (NO2–), nitric oxide (NO), nitrosamines and the effect of vitamin C. Nitric oxide (NO) is mainly responsible for the physiological effects of BRJ. The body uses arginine as a source to form NO, and for this reaction oxygen is needed. However, NO can also be formed after intake of nitrate-rich products such as BRJ. Ingested nitrate (NO3–) will be partly reduced to nitrite (NO2–) by microflora in the oral cavity. In oxygen-poor environments nitrate and nitrite can be reduced into NO. NO can also be oxidized back into nitrate and nitrite which are water soluble and can therefore be excreted in urine. Under acidic conditions, such as in the human stomach, nitrite will react with the H + and will form HNO2 (nitrous acid). Also in the stomach, two molecules HNO2 can form N2O3 (dinitrogen trioxide), by proton catalysis. N2O3 plays a role in the N-nitrosation rate. Increasing the amount of nitrate will therefore lead to an increase in the N-nitrosation rate. Subsequently, HNO2 can react with amides to form nitrosamides, and N2O3 can react with amines to form nitrosamines. Both nitrosamides and nitrosamines are N-nitroso compounds and potentially carcinogenic. Vitamin C can inhibit the nitrosation process, because it reacts faster than the amine with N2O3. Vitamin C reduces 2HNO2 to NO, and is itself oxidized to dehydroascorbic acid. This will reduce the amount of N-nitroso compounds that can be formed (Figure from Berends et al. (Citation2019) (Berends et al. Citation2019) with permission).