Figures & data
Figure 1. Graphical overview of the five main steps in the screening methodology of antimicrobial- producing insect symbionts. A popular method of identifying active insect symbionts and metabolites relies on microbial isolation and culturing, before performing an antimicrobial screening. In this traditional screening methodology, various key steps can be identified, indicated by the fields in various colours: isolation of insect symbionts, identification of isolates, fermentation and extraction, antimicrobial screening, and finally structure elucidation of active compounds. There is no fixed order when performing these steps, as indicated by the arrows.
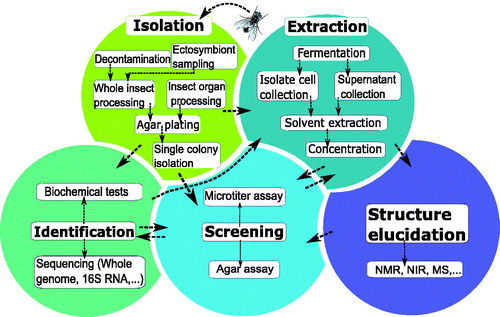
Figure 2. Orders and genera of insects of which microbiota are investigated for antimicrobial activity. The inner-circle represents the orders of the insects, the outer circle represents the insects’ genera.
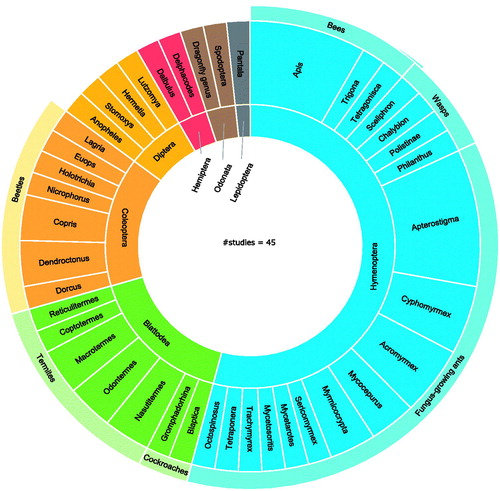
Figure 3. A graphical overview of the orders and genera of the insect symbionts with reported antimicrobial activity. Graph a shows the symbionts that have been found active through broad screening studies. Graph b shows active insect microbes that have been selected as symbiont of interest prior to antimicrobial screening. Graphs present microbes on genus level, and multiple species within the same genus were counted as one.
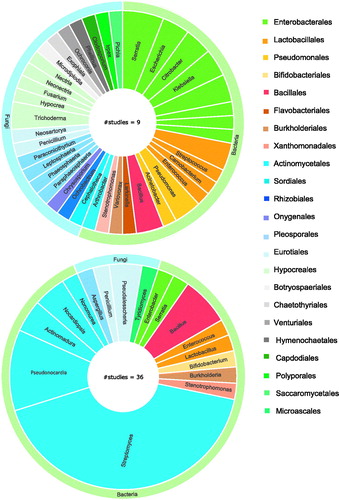
Figure 4. Overview of compounds with antimicrobial activity from insect symbionts. a) Amount of studies that isolated secondary metabolites from the symbiont(s) of interest. b) Visual representation of the structural classes the active molecules belong to.
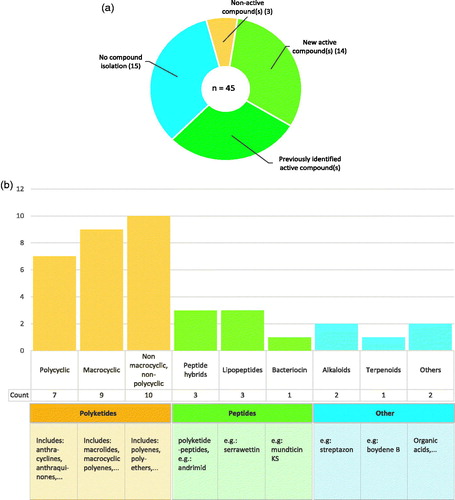
Table 1. Overview of compounds with antimicrobial activity isolated from insect symbiont strains.
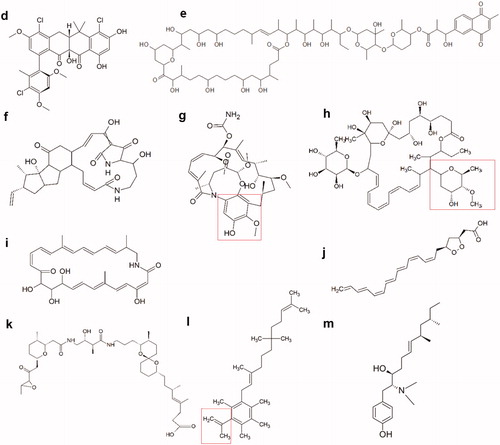
Figure 5.1. Molecular structures of new active peptides found in insect symbionts. (a) The cyclic lipodepsipeptide stephensiolide H. (b) Dentigerumycin, a polyketide-peptide hybrid. The polyketide fragment is indicated in red (c) The fungal toxin Boydine B, an epipolythiodioxopiperazine derived from a cyclic peptide.
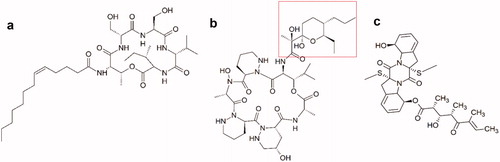
Figure 5.2. Molecular structures of new active polyketides found in insect symbionts. (d) Formicamycin F, one of the new pentacyclic polyketides. (e) Cyphomycin, a macrolide polyketide. (f) Frontalamide B, a polyketide with a macrolactam structure. (g) Natalamycin, a macrolactam polyketide. The substituted phenol function that replaces the benzoquinone function of the geldanamycins, is indicated in red. (h) Selvamicin, a polyene macrolide structurally very similar to the polyene antibiotics nystatin and amphotericin B. Indicated in red, is the sugar function that substitutes the amino sugar group of the regular polyene antibiotics. (i) Sceliphrolactam, a macrocyclic polyene. (j) Mycangimycin, a polyene peroxide with no macrocyclic ring structure. (k) Lagriamide, a polyether polyketide. (l) Ilicicolinic acid C. The carboxylic acid function is indicated in red. (m) N-methyltyroscherin, another non-polycyclic polyketide.