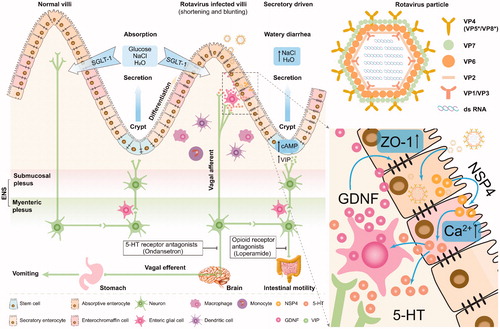
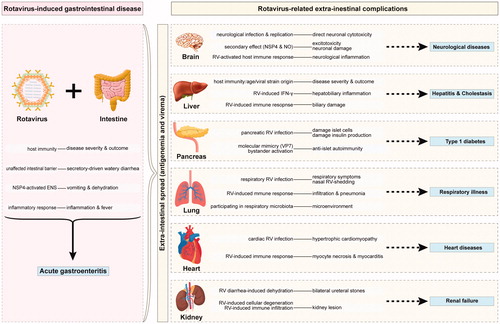
Figures & data
Table 1. Clinical manifestation and evidence summary of rotavirus-related systemic diseases.