Figures & data
Figure 1. Overview of the ECETOC and CLE Thyroid-NDT-TAS (see for details). BP: biocidal product; EDC-T: endocrine disruptor criteria for the thyroid modality; MoA: mode-of-action; PPP: plant protection product; REACH: Registration, Evaluation, Authorisation and Restriction of Chemicals; WoE: weight-of-evidence.
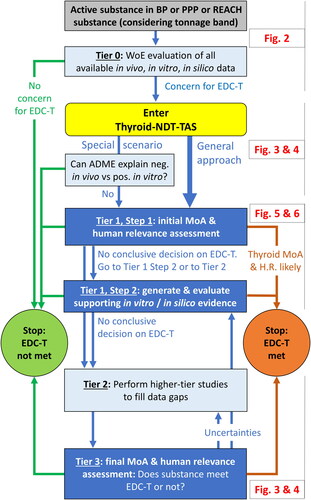
Figure 2. Tier 0: Evaluation of all available data to decide on the need to enter the ECETOC and CLE Thyroid-NDT-TAS.
EDC-T: endocrine disruptor criteria for thyroid modality; HP: histopathology; H.R.: human relevance; MoA: mode-of-action; MTD: maximum tolerated dose; T-modality: thyroid modality for endocrine disruption; TH: thyroid hormone; WoE: weight-of-evidence.
Colour legend: dark grey boxes: types of substances; light grey boxes, from right to left: production volumes (tonnage ranges) as per REACH Annexes VII-X, respectively; light blue boxes: elements of the assessment; blue arrows: continuation of evaluation; green arrows and text: negative findings; green circle: conclusion from Tier 0 evaluation that the EDC-T are not met. Yellow shape: conclusion from Tier 0 to enter the Thyroid-NDT-TAS.
[a] See Section 2.1.2.2 for elements to consider when applying expert judgement to determine whether the maximum tolerated dose was reached or exceeded.
[b] See Section 2.1.2.3 for elements to consider during the WoE evaluation of the in vivo thyroid-related findings.
[c] See Section 2.1.3 for elements to consider during the WoE evaluation of in vitro mechanistic data and to conclude that there is no evidence for in vitro activity.
[d] See Section 2.1.4 for elements to consider during the WoE evaluation of in silico data and to conclude that there is no evidence for in silico structural alerts.
[e] In vitro negative includes in vitro effects that only occurred at dose levels exceeding the in vivo top doses (as determined via in vitro-to-in vivo extrapolations).
[f] See Section 2.1.5 for aspects to consider in determining whether the in vivo database is sufficient. Inconsistent results in vivo vs. in vitro/in silico includes the scenarios “in vivo negative (in vivo database insufficient) combined with in vitro/in silico positive” and “in vivo positive combined with in vitro/in silico negative.”
[g] Respect information requirements for REACH substances depending on tonnage band and applicability of the European Commission (Citation2017, Citation2018) Endocrine Disruptor Criteria and EFSA and ECHA (Citation2018) Endocrine Disruptor Guidance.
![Figure 2. Tier 0: Evaluation of all available data to decide on the need to enter the ECETOC and CLE Thyroid-NDT-TAS.EDC-T: endocrine disruptor criteria for thyroid modality; HP: histopathology; H.R.: human relevance; MoA: mode-of-action; MTD: maximum tolerated dose; T-modality: thyroid modality for endocrine disruption; TH: thyroid hormone; WoE: weight-of-evidence.Colour legend: dark grey boxes: types of substances; light grey boxes, from right to left: production volumes (tonnage ranges) as per REACH Annexes VII-X, respectively; light blue boxes: elements of the assessment; blue arrows: continuation of evaluation; green arrows and text: negative findings; green circle: conclusion from Tier 0 evaluation that the EDC-T are not met. Yellow shape: conclusion from Tier 0 to enter the Thyroid-NDT-TAS.[a] See Section 2.1.2.2 for elements to consider when applying expert judgement to determine whether the maximum tolerated dose was reached or exceeded.[b] See Section 2.1.2.3 for elements to consider during the WoE evaluation of the in vivo thyroid-related findings.[c] See Section 2.1.3 for elements to consider during the WoE evaluation of in vitro mechanistic data and to conclude that there is no evidence for in vitro activity.[d] See Section 2.1.4 for elements to consider during the WoE evaluation of in silico data and to conclude that there is no evidence for in silico structural alerts.[e] In vitro negative includes in vitro effects that only occurred at dose levels exceeding the in vivo top doses (as determined via in vitro-to-in vivo extrapolations).[f] See Section 2.1.5 for aspects to consider in determining whether the in vivo database is sufficient. Inconsistent results in vivo vs. in vitro/in silico includes the scenarios “in vivo negative (in vivo database insufficient) combined with in vitro/in silico positive” and “in vivo positive combined with in vitro/in silico negative.”[g] Respect information requirements for REACH substances depending on tonnage band and applicability of the European Commission (Citation2017, Citation2018) Endocrine Disruptor Criteria and EFSA and ECHA (Citation2018) Endocrine Disruptor Guidance.](/cms/asset/9418836e-240e-4bec-9af4-de876e220e43/itxc_a_2231033_f0002_c.jpg)
Table 1. Overview of in vivo database that will generally be available for different types of substances during the Tier 0 evaluation.
Table 2. Measurements of serum T3, T4 and TSH in OECD test guidelines.
Table 3. Overview of in vitro assays, chemical methodologies and in silico models that allow investigating MIEs or key events of thyroid-related MoAs in mammals.
Figure 3. The ECETOC and CLE Thyroid-NDT-TAS. Scenario I: insufficient in vivo thyroid- and/or neurodevelopment-related data at onset of Tier 1.
ADME: absorption, distribution, metabolism, elimination; CTA: comparative thyroid assay; DNT: developmental neurotoxicity; EDC-T: endocrine disruptor criteria for thyroid modality; EOGRTS: extended one-generation reproductive toxicity study; H.R.: human relevance; MoA: mode-of-action; NDT: neurodevelopmental toxicity; OECD: Organisation for Economic Co-operation and Development; T3: triiodothyronine; T4: thyroxine; TG: test guideline; TK: toxicokinetics; TSH: thyroid stimulating hormone.
Colour legend: yellow shape: conclusion from Tier 0 evaluation to enter the Thyroid-NDT-TAS; dark blue boxes: MoA and human relevance assessment (); light blue boxes: elements of the assessment; blue arrows: continuation of evaluation; ochreous box and arrows: optional elements of the assessment (as the respective parameters have not yet been formally validated or adopted for regulatory use); grey shading: elements of the higher-tier testing; red-brown vs green arrows and text: findings leading to conclusion that the EDC-T are met (red-brown circle)/are not met (green circle).
[a] Consider offspring serum T4, T3, and TSH thresholds observed by Marty et al. (Citation2022) to support the determination of the biological relevance of findings (Section 2.1.2.3).
[b] Following expert judgement, further serum thyroid hormone data may not be necessary. Measurements of maternal and offspring plasma concentrations of the test material may be used to calculate placental transfer.
[c] See Section 2.2.3 for details on neurodevelopmental assessments.
[d] Following expert judgement, consider additional investigations using culled pups from the EOGRTS (or DNT study) and/or the performance of in vitro mechanistic assays and/or (not TG-conforming) perinatal studies to measure, e.g. brain thyroid hormones and/or receptor occupancies using immunohistochemistry. As relevant, consider measuring brain thyroid hormone already during the performance of the EOGRTS (or DNT study). Measurements of maternal and offspring plasma concentrations of the test material may be used to calculate placental transfer.
![Figure 3. The ECETOC and CLE Thyroid-NDT-TAS. Scenario I: insufficient in vivo thyroid- and/or neurodevelopment-related data at onset of Tier 1.ADME: absorption, distribution, metabolism, elimination; CTA: comparative thyroid assay; DNT: developmental neurotoxicity; EDC-T: endocrine disruptor criteria for thyroid modality; EOGRTS: extended one-generation reproductive toxicity study; H.R.: human relevance; MoA: mode-of-action; NDT: neurodevelopmental toxicity; OECD: Organisation for Economic Co-operation and Development; T3: triiodothyronine; T4: thyroxine; TG: test guideline; TK: toxicokinetics; TSH: thyroid stimulating hormone.Colour legend: yellow shape: conclusion from Tier 0 evaluation to enter the Thyroid-NDT-TAS; dark blue boxes: MoA and human relevance assessment (Figure 5); light blue boxes: elements of the assessment; blue arrows: continuation of evaluation; ochreous box and arrows: optional elements of the assessment (as the respective parameters have not yet been formally validated or adopted for regulatory use); grey shading: elements of the higher-tier testing; red-brown vs green arrows and text: findings leading to conclusion that the EDC-T are met (red-brown circle)/are not met (green circle).[a] Consider offspring serum T4, T3, and TSH thresholds observed by Marty et al. (Citation2022) to support the determination of the biological relevance of findings (Section 2.1.2.3).[b] Following expert judgement, further serum thyroid hormone data may not be necessary. Measurements of maternal and offspring plasma concentrations of the test material may be used to calculate placental transfer.[c] See Section 2.2.3 for details on neurodevelopmental assessments.[d] Following expert judgement, consider additional investigations using culled pups from the EOGRTS (or DNT study) and/or the performance of in vitro mechanistic assays and/or (not TG-conforming) perinatal studies to measure, e.g. brain thyroid hormones and/or receptor occupancies using immunohistochemistry. As relevant, consider measuring brain thyroid hormone already during the performance of the EOGRTS (or DNT study). Measurements of maternal and offspring plasma concentrations of the test material may be used to calculate placental transfer.](/cms/asset/e402007b-134f-45ed-9851-dc21fb965417/itxc_a_2231033_f0003_c.jpg)
Figure 4. The ECETOC and CLE Thyroid-NDT-TAS: Scenario II: sufficient in vivo thyroid- and neurodevelopment-related data at onset of Tier 1.
DNT: developmental neurotoxicity; EDC-T: endocrine disruptor criteria for thyroid modality; EOGRTS: extended one-generation reproductive toxicity study; H.R.: human relevance; MoA: mode-of-action; NDT: neurodevelopmental toxicity; T3: triiodothyronine; T4: thyroxine; TG: test guideline.
Colour legend: yellow shape: conclusion from Tier 0 evaluation to enter the Thyroid-NDT-TAS; light blue boxes: elements of the assessment; blue arrows: continuation of evaluation; ochreous box and arrows: optional elements of the assessment (as the respective parameters have not yet been formally validated or adopted for regulatory use); dotted ochreous arrow: expert judgement that offspring brain thyroid hormones and/or further neurodevelopmental parameters are relevant to substantiate or rule out NDT; dark blue box: MoA and human relevance assessment (); red-brown vs green arrows and text: findings leading to conclusion that the EDC-T are met (red-brown circle)/are not met (green circle).
[a] See Section 2.2.3 for details on neurodevelopmental assessments.
[b] Following expert judgement, consider additional investigations using culled pups from the EOGRTS and/or the performance of in vitro mechanistic assays and/or (not TG-conforming) perinatal studies to measure e.g. brain thyroid hormones and/or receptor occupancies using immunohistochemistry. Measurements of maternal and offspring plasma concentrations of the test material may be used to calculate placental transfer.
![Figure 4. The ECETOC and CLE Thyroid-NDT-TAS: Scenario II: sufficient in vivo thyroid- and neurodevelopment-related data at onset of Tier 1.DNT: developmental neurotoxicity; EDC-T: endocrine disruptor criteria for thyroid modality; EOGRTS: extended one-generation reproductive toxicity study; H.R.: human relevance; MoA: mode-of-action; NDT: neurodevelopmental toxicity; T3: triiodothyronine; T4: thyroxine; TG: test guideline.Colour legend: yellow shape: conclusion from Tier 0 evaluation to enter the Thyroid-NDT-TAS; light blue boxes: elements of the assessment; blue arrows: continuation of evaluation; ochreous box and arrows: optional elements of the assessment (as the respective parameters have not yet been formally validated or adopted for regulatory use); dotted ochreous arrow: expert judgement that offspring brain thyroid hormones and/or further neurodevelopmental parameters are relevant to substantiate or rule out NDT; dark blue box: MoA and human relevance assessment (Figure 5); red-brown vs green arrows and text: findings leading to conclusion that the EDC-T are met (red-brown circle)/are not met (green circle).[a] See Section 2.2.3 for details on neurodevelopmental assessments.[b] Following expert judgement, consider additional investigations using culled pups from the EOGRTS and/or the performance of in vitro mechanistic assays and/or (not TG-conforming) perinatal studies to measure e.g. brain thyroid hormones and/or receptor occupancies using immunohistochemistry. Measurements of maternal and offspring plasma concentrations of the test material may be used to calculate placental transfer.](/cms/asset/99970a67-28a3-4e49-858b-c7635b27df98/itxc_a_2231033_f0004_c.jpg)
Figure 5. Decision-tree for MoA and human relevance assessment embedded in Tier 1 and Tier 3 of the ECETOC and CLE Thyroid-NDT-TAS.
DIO: deiodinase; EDC-T: endocrine disruptor criteria for thyroid modality; H.R.: human relevance; LEI: liver enzyme induction; MoA: mode-of-action; NIS: sodium – iodide symporter; SBP: serum binding protein; T3: triiodothyronine; T4: thyroxine; TH: thyroid hormone; TPO: thyroid peroxidase; TR: thyroid receptor (nuclear); TSH: thyroid stimulating hormone; UGT: uridine diphosphate glucuronyltransferase.
Colour legend: dark blue boxes: Step 1 and Step 2 of the MoA and human relevance assessment; light blue boxes: elements of the assessment; blue arrows: continuation of evaluation; dotted blue arrow: expert judgement that Step 2 of Tier 1 should be skipped to directly continue to Tier 2 to generate higher-tier data; light green boxes: optional elements of the assessment as the respective parameters have not yet been formally adopted for regulatory use; ochreous box: optional assessment as the corresponding MIEs seem to be less frequent; red-brown vs green arrows and text: findings leading to conclusion that the EDC-T are met (red-brown circle)/are not met (green circle). Yellow shape: continuation of Thyroid-NDT-TAS.
[a] TSH likely not increased, and no thyroid organ changes, if only in utero/developmental exposure to substances enhancing thyroid hormone clearance (Marty et al. Citation2022).
[b] See Section 2.3.1 for elements to consider during the WoE evaluation.
[c] Apply expert judgement to determine which type of supporting in vitro and/or in silico evidence may be relevant for the substance of interest.
[d] Primarily TPO and NIS inhibition need to be excluded, as thyroid-related parameters are similarly affected by substances acting via a direct thyroid-related MoA. Also, depending on the thyroid hormone effect pattern (e.g. increased serum T4), substance interaction with DIOs needs to be excluded.
[e] The final MoA and human relevance assessment shall serve to answer the questions: Is the adverse effect not a consequence of thyroid MoA? If the substance has a thyroid MoA, is it not relevant for humans?
![Figure 5. Decision-tree for MoA and human relevance assessment embedded in Tier 1 and Tier 3 of the ECETOC and CLE Thyroid-NDT-TAS.DIO: deiodinase; EDC-T: endocrine disruptor criteria for thyroid modality; H.R.: human relevance; LEI: liver enzyme induction; MoA: mode-of-action; NIS: sodium – iodide symporter; SBP: serum binding protein; T3: triiodothyronine; T4: thyroxine; TH: thyroid hormone; TPO: thyroid peroxidase; TR: thyroid receptor (nuclear); TSH: thyroid stimulating hormone; UGT: uridine diphosphate glucuronyltransferase.Colour legend: dark blue boxes: Step 1 and Step 2 of the MoA and human relevance assessment; light blue boxes: elements of the assessment; blue arrows: continuation of evaluation; dotted blue arrow: expert judgement that Step 2 of Tier 1 should be skipped to directly continue to Tier 2 to generate higher-tier data; light green boxes: optional elements of the assessment as the respective parameters have not yet been formally adopted for regulatory use; ochreous box: optional assessment as the corresponding MIEs seem to be less frequent; red-brown vs green arrows and text: findings leading to conclusion that the EDC-T are met (red-brown circle)/are not met (green circle). Yellow shape: continuation of Thyroid-NDT-TAS.[a] TSH likely not increased, and no thyroid organ changes, if only in utero/developmental exposure to substances enhancing thyroid hormone clearance (Marty et al. Citation2022).[b] See Section 2.3.1 for elements to consider during the WoE evaluation.[c] Apply expert judgement to determine which type of supporting in vitro and/or in silico evidence may be relevant for the substance of interest.[d] Primarily TPO and NIS inhibition need to be excluded, as thyroid-related parameters are similarly affected by substances acting via a direct thyroid-related MoA. Also, depending on the thyroid hormone effect pattern (e.g. increased serum T4), substance interaction with DIOs needs to be excluded.[e] The final MoA and human relevance assessment shall serve to answer the questions: Is the adverse effect not a consequence of thyroid MoA? If the substance has a thyroid MoA, is it not relevant for humans?](/cms/asset/fcf8e43d-8c90-40e1-95bd-4ea7e3f52212/itxc_a_2231033_f0005_c.jpg)
Figure 6. Sequence of events that may lead from liver enzyme induction (AOP 8) and interaction with serum binding proteins (AOP 152) to adverse neurodevelopmental outcomes in mammals and opportunities for their further investigation.
AhR: aryl hydrocarbon receptor; AO(P): adverse outcome (pathway); BROD: benzoxyresorufin; CAR: constitutive androstane receptor; Cyp: cytochrome p-450; fT4: free thyroxine; H.R.: human relevance; KE: key event; LDG: lower-dose groups; MIE: molecular initiating event; NIS: sodium – iodide symporter; PBK: physiologically based kinetic; PPAR: peroxisome proliferator-activated receptor; PROD: pentoxyresorufin; PXR: pregnane X receptor; SBP: serum binding protein; T3: triiodothyronine; T4: thyroxine; TDG: top-dose group; TSH: thyroid stimulating hormone; TTR: transthyretin; UGT: uridine diphosphate glucuronyltransferase.
Colour legend: yellow arrow: available data indicating that AOP 8/AOP 152 may be relevant for the MoA and human relevance assessment. Boxes with blue/red-brown shading: MIE, early KEs and AOs that relate to AOP 8/AOP 152. White boxes with blue/red-brown text: Supportive in vivo or in vitro evidence that may inform on the MIE or specific KEs for AOP 8/AOP 152 (linked by blue/red-brown arrows; black arrow for enhanced traceability). Boxes with green shading: KEs relating to serum/brain T4 decrements; these KEs are central to five of the six potentially relevant AOPs included in the OECD AOP Wiki (exception: AOP 300 on thyroid receptor antagonism; ). White box with green text: In vivo data or PBK modelling to inform on thyroid-related events. Boxes with grey shadings: KEs relating to the hippocampus; these KEs are central to five of the six potentially relevant AOPs (exception: AOP 54 on NIS inhibition leading to impaired learning and memory). Dotted black arrow: T4 measurements in relevant tissues, if available.
[a] In the OECD AOP Wiki, the MIE of AOP 8 is recorded as PXR activation. Noyes et al. (Citation2019) indicated CAR, AhR and PPAR activation as further MIEs leading to liver enzyme induction. The ECETOC T4 TF and CLE contend that all these MIEs are not indispensable to trigger UGT upregulation. Therefore, assessments addressing hepatic nuclear receptor activation may not be needed for the MoA assessment of the substance of interest.
[b] While AOP 152 only refers to TTR, the available in vitro assays generally allow measuring all three major serum binding proteins, i.e. TTR, albumin and thyroid binding globulin. Since thyroid hormone distribution across these three serum binding proteins and their binding affinities differ considerably between rats and humans, the ECETOC T4 TF and CLE recommend considering in vitro substance interaction with all three serum binding proteins, as relevant.
[c] See Tinwell and Bars (Citation2022) for details on the indirect assessment of CAR/PXR activation in rat studies via induction of transcript level and corresponding enzyme activity associated with each receptor (Cyp2b/PROD and Cyp3a/BROD for CAR and PXR, respectively).
[d] PBK modelling: Estimate serum/brain T4 levels in rat vs human considering relevant parameters, such as binding constants, potencies of MIEs and/or liver enzyme inductions in rat vs human tissue.
[e] The AOPs in the OECD AOP Wiki () only generally refer to “T4 in serum, decrease” without distinction between maternal and offspring serum T4 levels; also, none of the AOPs considers serum T3 (or TSH). Following the observations by Marty et al. (Citation2022), maternal serum T4 levels do not appear predictive of neurodevelopmental effects. However, there seems to be some association between ≥ 60%/≥ 50% offspring serum T4 decrements in the TDG/LDGs (and ≥ 20% and statistically significant offspring serum T3 decrements) and the occurrence of statistically significant neurodevelopmental effects. Therefore, the ECETOC T4 TF and CLE recommend considering offspring serum T4 as predominant parameter related to serum thyroid hormone levels. In addition, information on maternal serum T4, maternal and/or offspring serum T3 and offspring brain T4/T3 should be considered, if available (see Section 2.1.2.3 for further discussion).
[f] For AOP 152 (as well as AOP 42 and AOP 134), “cochlear function, decreased/loss” was indicated as adverse outcome in the OECD AOP Wiki as per 13 September 2019, whereas it was indicated as “cognitive function, decreased” as per 15 October 2019.
![Figure 6. Sequence of events that may lead from liver enzyme induction (AOP 8) and interaction with serum binding proteins (AOP 152) to adverse neurodevelopmental outcomes in mammals and opportunities for their further investigation.AhR: aryl hydrocarbon receptor; AO(P): adverse outcome (pathway); BROD: benzoxyresorufin; CAR: constitutive androstane receptor; Cyp: cytochrome p-450; fT4: free thyroxine; H.R.: human relevance; KE: key event; LDG: lower-dose groups; MIE: molecular initiating event; NIS: sodium – iodide symporter; PBK: physiologically based kinetic; PPAR: peroxisome proliferator-activated receptor; PROD: pentoxyresorufin; PXR: pregnane X receptor; SBP: serum binding protein; T3: triiodothyronine; T4: thyroxine; TDG: top-dose group; TSH: thyroid stimulating hormone; TTR: transthyretin; UGT: uridine diphosphate glucuronyltransferase.Colour legend: yellow arrow: available data indicating that AOP 8/AOP 152 may be relevant for the MoA and human relevance assessment. Boxes with blue/red-brown shading: MIE, early KEs and AOs that relate to AOP 8/AOP 152. White boxes with blue/red-brown text: Supportive in vivo or in vitro evidence that may inform on the MIE or specific KEs for AOP 8/AOP 152 (linked by blue/red-brown arrows; black arrow for enhanced traceability). Boxes with green shading: KEs relating to serum/brain T4 decrements; these KEs are central to five of the six potentially relevant AOPs included in the OECD AOP Wiki (exception: AOP 300 on thyroid receptor antagonism; Table Appendix 3). White box with green text: In vivo data or PBK modelling to inform on thyroid-related events. Boxes with grey shadings: KEs relating to the hippocampus; these KEs are central to five of the six potentially relevant AOPs (exception: AOP 54 on NIS inhibition leading to impaired learning and memory). Dotted black arrow: T4 measurements in relevant tissues, if available.[a] In the OECD AOP Wiki, the MIE of AOP 8 is recorded as PXR activation. Noyes et al. (Citation2019) indicated CAR, AhR and PPAR activation as further MIEs leading to liver enzyme induction. The ECETOC T4 TF and CLE contend that all these MIEs are not indispensable to trigger UGT upregulation. Therefore, assessments addressing hepatic nuclear receptor activation may not be needed for the MoA assessment of the substance of interest.[b] While AOP 152 only refers to TTR, the available in vitro assays generally allow measuring all three major serum binding proteins, i.e. TTR, albumin and thyroid binding globulin. Since thyroid hormone distribution across these three serum binding proteins and their binding affinities differ considerably between rats and humans, the ECETOC T4 TF and CLE recommend considering in vitro substance interaction with all three serum binding proteins, as relevant.[c] See Tinwell and Bars (Citation2022) for details on the indirect assessment of CAR/PXR activation in rat studies via induction of transcript level and corresponding enzyme activity associated with each receptor (Cyp2b/PROD and Cyp3a/BROD for CAR and PXR, respectively).[d] PBK modelling: Estimate serum/brain T4 levels in rat vs human considering relevant parameters, such as binding constants, potencies of MIEs and/or liver enzyme inductions in rat vs human tissue.[e] The AOPs in the OECD AOP Wiki (Table Appendix 3) only generally refer to “T4 in serum, decrease” without distinction between maternal and offspring serum T4 levels; also, none of the AOPs considers serum T3 (or TSH). Following the observations by Marty et al. (Citation2022), maternal serum T4 levels do not appear predictive of neurodevelopmental effects. However, there seems to be some association between ≥ 60%/≥ 50% offspring serum T4 decrements in the TDG/LDGs (and ≥ 20% and statistically significant offspring serum T3 decrements) and the occurrence of statistically significant neurodevelopmental effects. Therefore, the ECETOC T4 TF and CLE recommend considering offspring serum T4 as predominant parameter related to serum thyroid hormone levels. In addition, information on maternal serum T4, maternal and/or offspring serum T3 and offspring brain T4/T3 should be considered, if available (see Section 2.1.2.3 for further discussion).[f] For AOP 152 (as well as AOP 42 and AOP 134), “cochlear function, decreased/loss” was indicated as adverse outcome in the OECD AOP Wiki as per 13 September 2019, whereas it was indicated as “cognitive function, decreased” as per 15 October 2019.](/cms/asset/49b4e495-98eb-46c7-98c7-c09763f678aa/itxc_a_2231033_f0006_c.jpg)
Table Appendix 1. Possible scenarios for the Tier 0 in vivo thyroid- and neurodevelopment-related database.
Table Appendix 2. Research recommendations to enhance assessments of thyroid function and neurodevelopmental impairment (adapted from Marty et al. Citation2022).
Table Appendix 3. Thyroid-related AOPs including neurodevelopmental outcomes in mammals listed in the OECD AOP Wiki in May 2023 (adapted from Marty et al. Citation2021).
