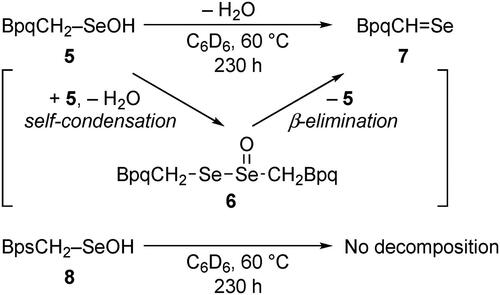
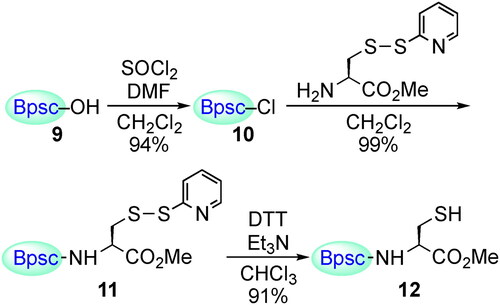
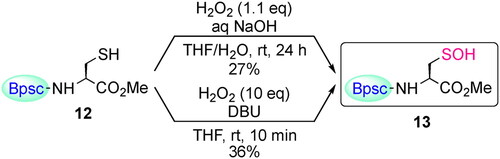
Figures & data
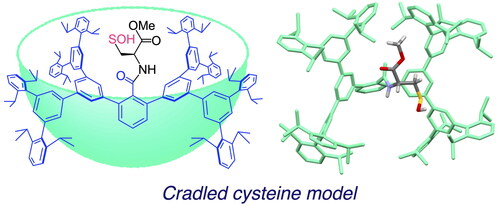
Figure 1. Examples of isolable non-cysteinyl R–SOH. Thermodynamically stabilized compound (1)[Citation17] and kinetically stabilized ones (2,[Citation8] 3,[Citation13] and 4[Citation15]).
![Figure 1. Examples of isolable non-cysteinyl R–SOH. Thermodynamically stabilized compound (1)[Citation17] and kinetically stabilized ones (2,[Citation8] 3,[Citation13] and 4[Citation15]).](/cms/asset/113e1222-8eb4-4935-95f0-25b1d91978b0/gpss_a_2195650_f0001_b.jpg)
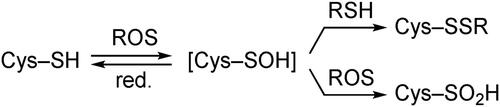
Figure 3. Cradled cysteine model for stabilization of cysteine-derived reactive intermediates such as Cys–SOH.
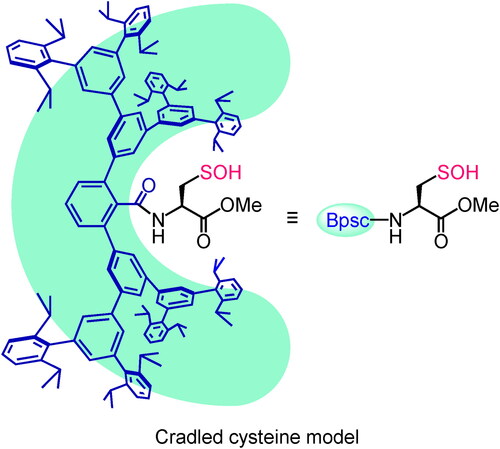
Figure 4. Crystal structure of Cys–SOH 13: (a) stick representation (hydrogen atoms of the Bpsc group are omitted for clarity), and (b) space-filling representation.
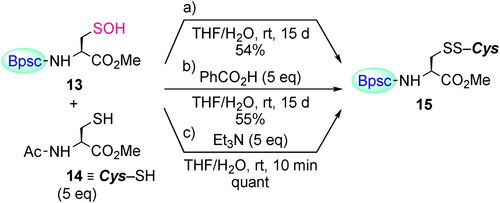
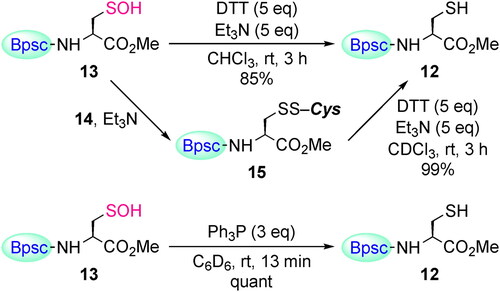
Scheme 6. Reaction of Cys–SOH 13 with thiol 14. The yields of 15 were estimated by no-D 1H NMR spectroscopy utilizing 1,3,5-trimethoxybenzene as an internal standard.