Figures & data
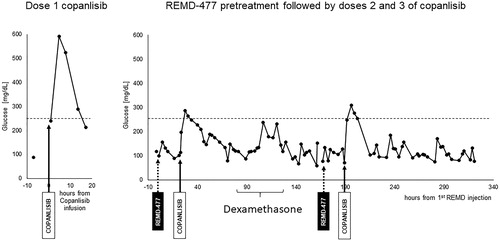
Figure 1. Blood glucose level and change of body weight in CB17 SCID mice treated with copanlisib with or without REMD 2.59c. Healthy female CB17 SCID mice, aged 6–8 weeks with body weight of normal range (20–21 g), were included in the study. Mice had free access to sterilized dry granule food and water during the entire study period. (A) Mice received vehicle or copanlisib (15 mg/kg) via tail vein injection every other day (days 1, 3, 5, 7, and 9). (B) As in A, mice received intravenous administration of copanlisib every other day (days 1, 3, 5, 7, 9 and 11). The mice also received intraperitoneal injection of vehicle or REMD2.59c mAb (7 mg/kg) on days 0, 4, 8 and 10. (C) Blood glucose levels were measured by tail vein nick using an Accu-ChekTM glucometer 2h after dosing (days 1, 5 and 9) and 2 days after final dosing (day 11) of copanlisib. Data are shown as mean ± SEM. (D) As in C, blood glucose levels were measured 2h after dosing (days 1, 3, 9 and 11). Student’s t-test was performed for the comparison of blood glucose levels. p value: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (E, F) Body weights of all animals were measured throughout the study. Body weight change, expressed in %, was calculated using the formula: BW change (%) = (BW Day x/BW Day 0) × 100%. Data are shown as mean ± SEM.
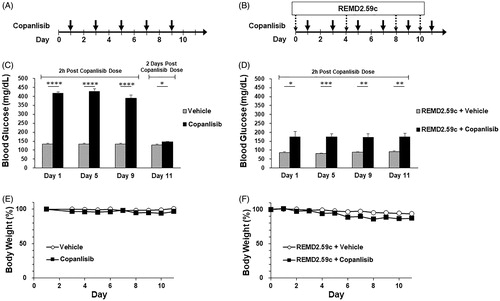
Figure 2. Blood glucose levels in a patient treated with copanlisib without REMD-477 and then with REMD-477. A patient with relapsed/refractory peripheral T cell lymphoma (PTCL) NOS treated with 60mg IV copanlisib alone experienced hyperglycemia as shown in the left panel. The patient provided a signed informed consent to participate in an IRB-approved clinical pilot study of REMD-477 plus copanlisib. The patient received 70mg REMD-477 subcutaneously (SQ) 1 day before copanlisib in weeks one and two. Blood glucose was measured at selected time points and shown in the right panel. Solid arrows indicate the time of copanlisib administration, and dashed arrows indicate administration of REMD-477.