Figures & data
Table 1. Summary of the applied models. VE: Viable epidermis, SC: Stratum corneum, RF: Receptor fluid.
Figure 1. Relation of the experimental (logExperimentalAbsorption) to the predicted absorption (logPredictedAbsorption). The experimental absorption was taken as the sum of the relative amount present in the receptor fluid, the skin sample and the tape strips – except for the first and second ones. The predicted absorption represents the absorption as calculated by each model. The diagonal line represents the function y = x.
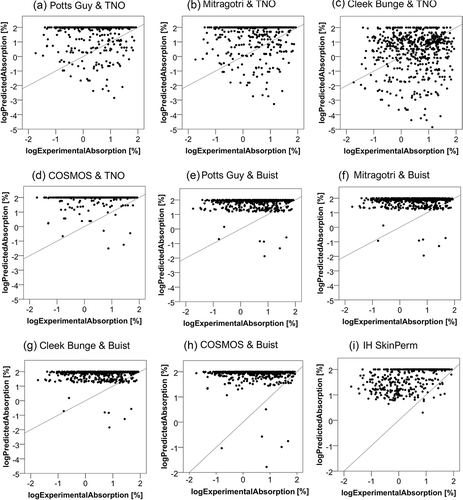
Table 2. Best performing models ranked according to the four criteria.
Figure 2. Relation of the prediction factor to the active substance’s lipophilicity (log kow). Dermal absorption was predicted using the models indicated in the figure and prediction factors (PF) were calculated by dividing by experimental values. The logarithm (log PF) is plotted against measured octanol-water partition coefficients (log kow). In figures (a,c) open circles represent the cases out of the applicability domain. The horizontal lines mark the limit, under which the predicted is lower than the experimental absorption and the vertical the limit under which a substance is considered hydrophilic.
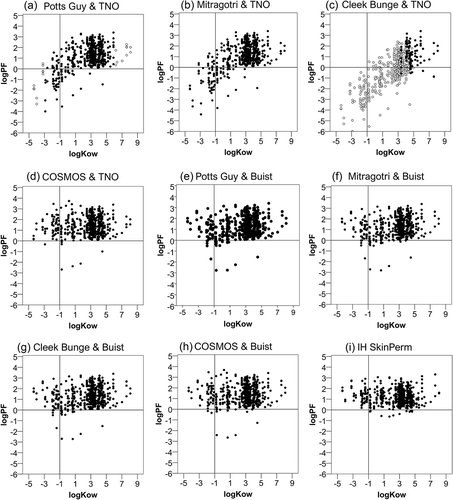
Figure 3. Relation of the prediction factor to the active substance’s molecular weight. Dermal absorption was predicted using the models indicated in the figure and prediction factors (PF) were calculated by dividing by experimental values. The logarithm (log PF) is plotted against the molecular weight. In figures (a and c) open circles represent the cases out of the applicability domain. The horizontal lines mark the limit, under which the predicted is lower than the experimental absorption.
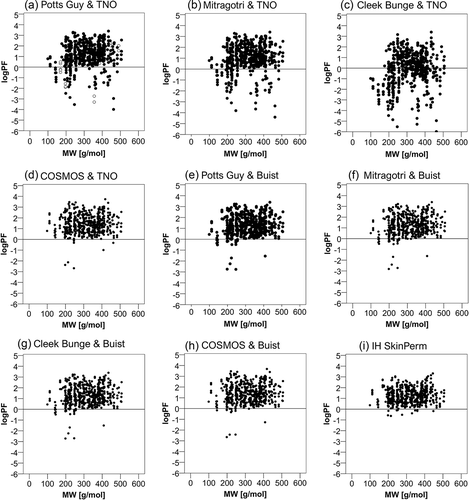
Table 3. Summary of the Pearson correlation coefficients calculated for the relationship between the log PF (log prediction factor) and lipophilicity (expressed as log kow) or molecular weight (MW). AD: values within applicability domain included only.
Figure 4. Distribution of the prediction factor (log PF) in different physical states. The left whisker marks the minimum value and is defined as 1.5xIQR, the left part of the box represents the 1st quartile, the middle vertical line in the box represents the median, the right part of the box represents the 3rd quartile, and the right whisker the maximum value, defined as 1.5xIQR. The circles mark the outliers and the asterisks the extremes. The vertical line in the diagram marks the limit, under which the predicted is lower than the experimental absorption. Δ marks statistically significant differences to liquids; Π to pastes, as identified by non-parametric testing performed within each model, with alpha value set to 0.05.
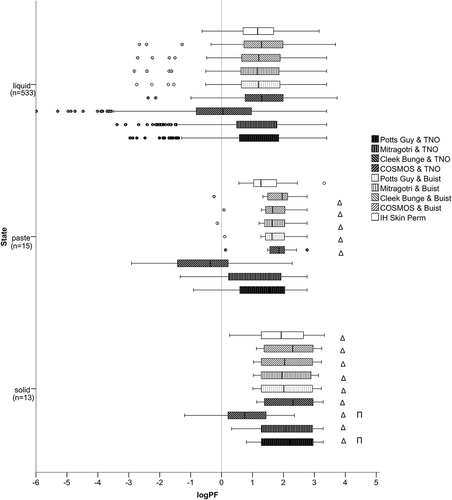
Table 4. Summary of the examined factors: expected vs. observed outcomes on the predictivity of the models.
Table 5. Applicability of the models as a refinement alternative to the current EFSA default values. The number of cases for which the predicted absorption would be lower than the default values as applicable according to the EFSA guidance [Citation5] was counted. When, for those cases, predicted absorption was below experimental absorption, these were classified as refinement underestimates.
