Figures & data
Table 1. Composition (%, w/v) of unloaded NLC and Myr-NLC formulations.
Table 2. Characteristics of NLC formulations.
Table 3. Stability of NLC and Myr-NLC formulations.
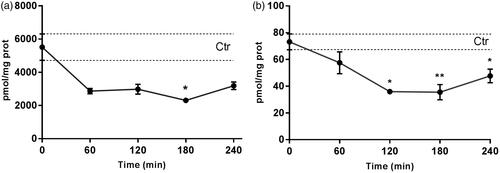
Figure 1. Myriocin (topically administered as a myriocin-NLC) distribution in rabbit vitreous and retina. *p < .05 mouse retina vs. rabbit vitreous and retina. **p < .05 rabbit vitreous vs. rabbit retina.
Table 4. Ocular PK parameters of the myriocin-NLC (NLC1) formulation.