Figures & data
Figure 1. Basic concept of drug uptake from ASDs. From the solid state of ASDs (drug-rich particles of pure drug (ALPS), drug-rich particles containing polymers, micelles, and crystals), a complex mixture of API in solution and colloidal API emerges, from which the drug uptake through the intestinal membrane is induced.
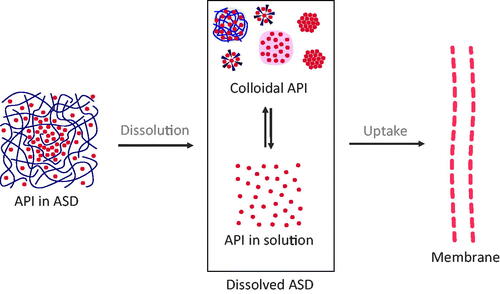
Figure 2. Classification of physicochemical concepts of solubility, supersaturation, and solubilization. Two equilibria of API in solution can be differentiated: (1) the equilibrium between crystalline API and API in solution is referred to the crystalline solubility and (2) the equilibrium between API in amorphous liquid phase separation (ALPS) and API in solution. Compared to equilibrium 1, equilibrium 2 shows a higher concentration of molecularly dissolved drug (referred to as supersaturation). In contrast, solubilization, e.g. by surfactants, does not lead to an increased concentration of molecularly dissolved drug.
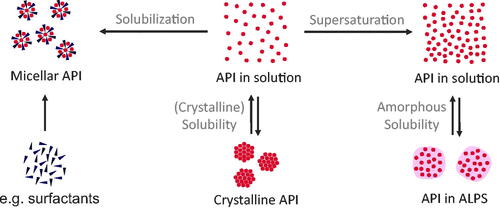
Figure 3. Three main concepts for dissolution from ASDs. (1) In the case of carrier controlled release, drug molecules have to diffuse through the polymer, possibly through a highly viscous gel layer on the surface of ASD particles. If dissolved drug concentrations become high enough to exceed the amorphous solubility, ALPS will occur, inducing the formation of drug-rich particles. (2) In the case of dissolution controlled release, API and polymer dissolve congruently, leading to fast dissolution and formation of drug-rich particles. The polymer may stabilize the supersaturated solution. (3) In the case of drug controlled release, the polymer dissolves out of the ASD and the residual API controls the dissolution rate. If the residual API is not stable in the amorphous state without polymer, i.e. crystallizes, supersaturation will not occur.
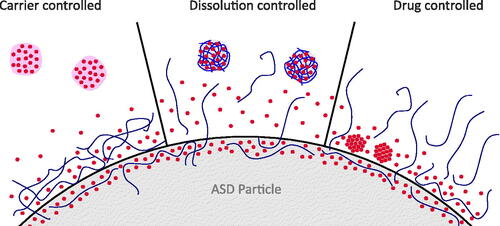
Figure 4. Classifications of states within dissolved ASDs. Dissolved API is in equilibrium with crystalline API or API in ALPS. The supersaturated state and, if the amorphous solubility is exceeded, ALPS are in a metastable state, where crystallization can occur (cryst.). Crystallization will lead to a reduction in concentration of the dissolved state down to crystalline solubility. Furthermore, there is an equilibrium between solubilized API and both dissolved states of dissolved API, i.e. the supersaturated and the not supersaturated state.
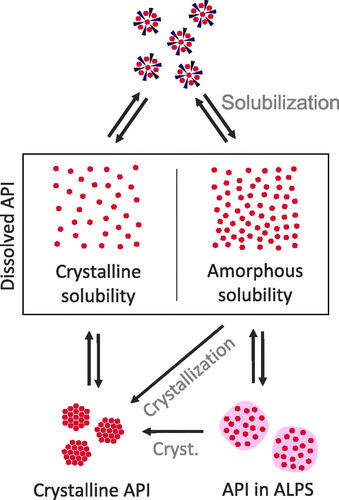
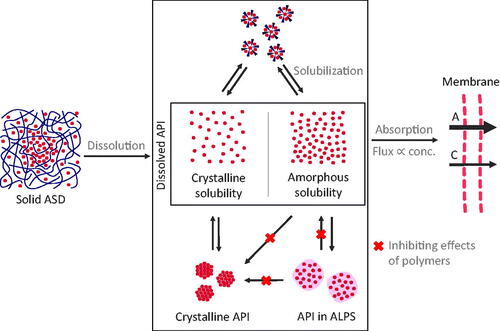
Figure 5. Scheme of the overall concept of increased bioavailability through ASDs. Upon dissolution of the solid ASD, a complex system of dissolved stated of ASDs emerges. In this system, solubilizing excipients can reduce the concentration of dissolved API. Supersaturation can be stabilized by polymers, however, polymers can also reduce the concentration of the molecularly dissolved API in favor of an increased fraction of ALPS. An increase in the molecularly dissolved drug concentration (conc.) from crystalline solubility (C) to amorphous solubility (A) leads to an increased transmembrane flux and therefore improved absorption.