Figures & data
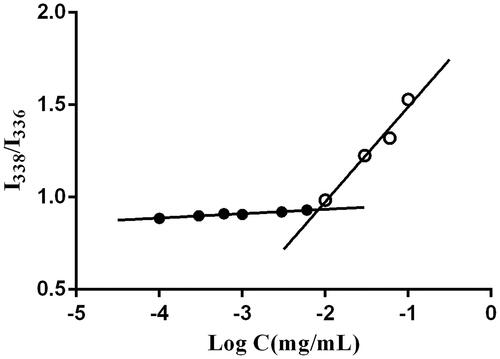
Figure 1. Effects of the ratio of soluplus and TPGS on the (A) particle size and PDI, (B) encapsulation efficiency and drug leakage percent, (C) precipitation in simulated gastrointestinal tract of DE-CMs (n = 3). PDI: polydispersity index; DE-CMs: dabigatran etexilate-loaded composite micelles.
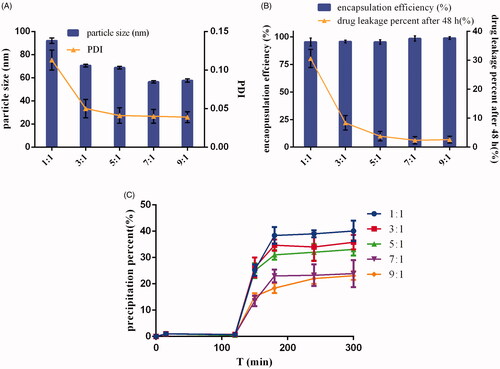
Figure 2. (A) Particle size distribution and (B) TEM image of DE-CMs. TEM: transmission electron microscope; DE-CMs: dabigatran etexilate-loaded composite micelles.
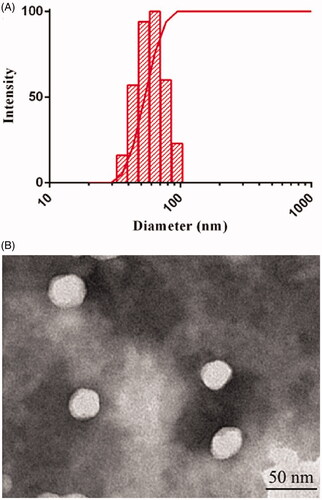
Figure 3. (A) DSC analyses and (B) XRD analyses of DE, blank composite micelles and DE-CMs. DSC: differential scanning calorimetry; XRD: X-ray powder diffractometry; DE: dabigatran etexilate; DE-CMs: dabigatran etexilate-loaded composite micelles.
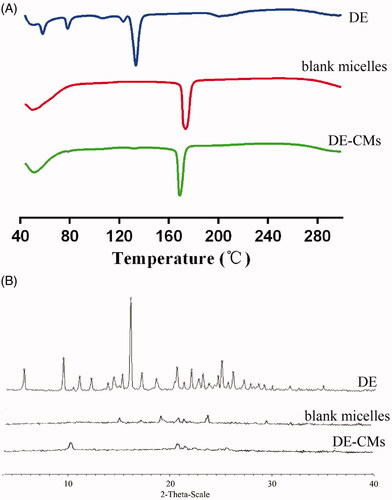
Figure 5. (A) In vitro formation and transport process of DE-CMs in the GI tract. (B) Percentage of free drugs and drugs in DE-CMs after incubation in SGF, SIF and distilled water under 37 °C for 2 h. (C) In vitro precipitation of the commercial formulation, DE-CMs, mixture (DE, soluplus and TPGS) under SGIF (n = 3). DE-CMs: dabigatran etexilate-loaded composite micelles; SGF: simulated gastric fluid; SIF: simulated intestinal fluid; SGIF: simulated gastrointestinal fluid.
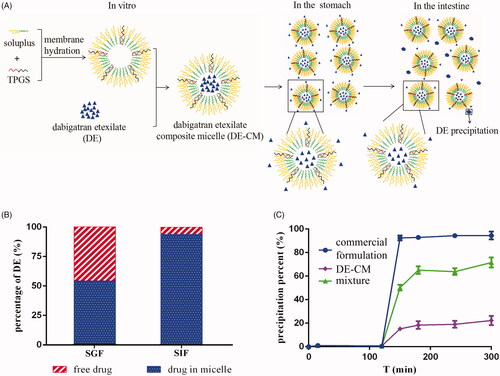
Figure 6. (A) Cell viability of DE, DE-SMs and DE-CMs on Caco-2 cells (n = 3). (B) Permeability of DE, DE-SMs and DE-CMs across Caco-2 cell monolayer (n = 3). *p < .05, **p < .01 versus DE; #p < .05, ##p < .01 versus DE-SMs. DE: dabigatran etexilate; DE-SMs: dabigatran etexilate-loaded single micelles; DE-CMs: dabigatran etexilate-loaded composite micelles; AP-BL: from apical to basolateral; BL-AP: from basolateral to apical.
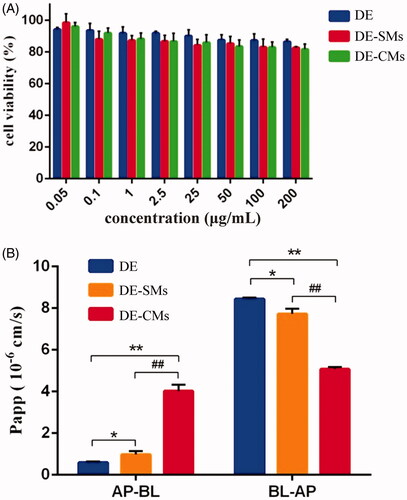
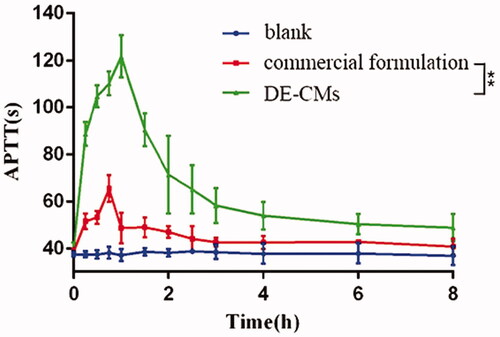
Figure 7. Dabigatran plasma concentration-time of the commercial formulation and DE-CMs in rats (n = 6). **p < .01 versus the commercial formulation. DE-CMs: dabigatran etexilate-loaded composite micelles.
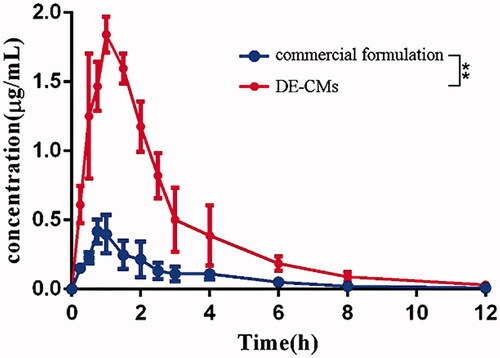
Table 1. Pharmacokinetic parameters of dabigatran etexilate in rats after oral administration of commercial formulation and DE-CMs (mean ± SD, n = 6).