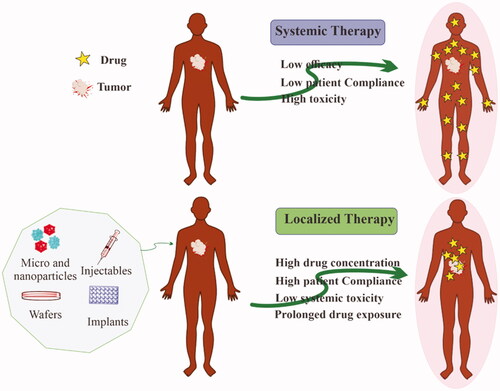
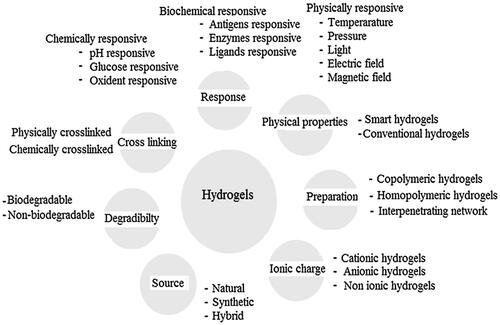
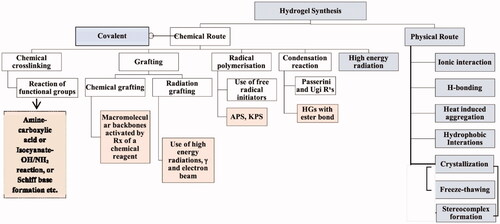
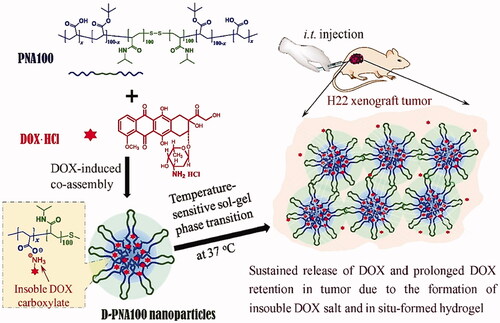
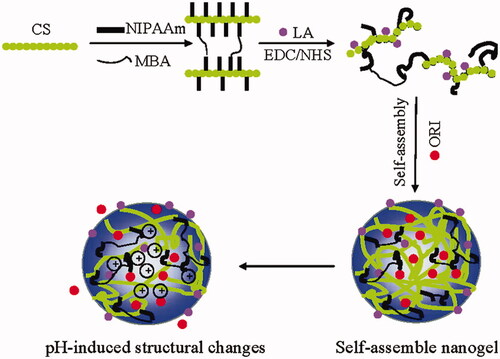
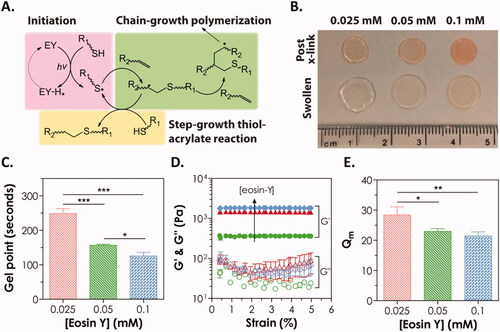
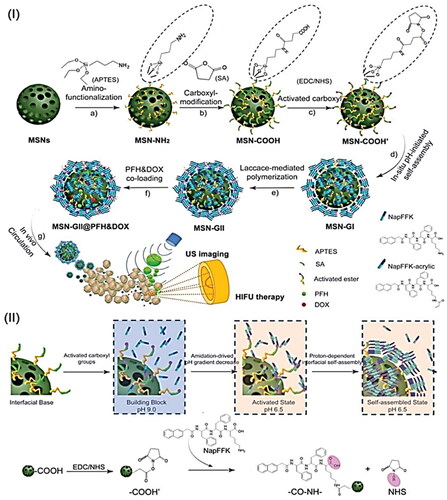
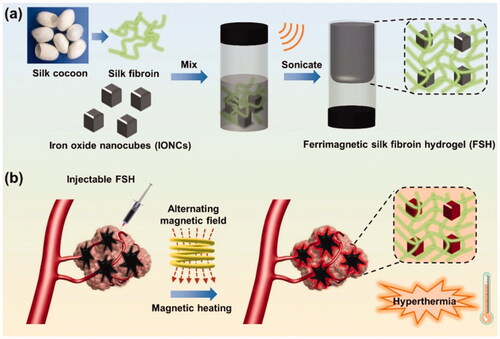
Figures & data
Table 1. Hydrogel-based drug delivery systems for cancer treatment.
Table 2. List of stimuli-responsive hydrogel for liver cancer therapy.
Table 3. Hydrogel-based active targeting and combination therapy for liver cancer therapy.
Table 4. Advantages and shortcomings of different hydrogel delivery routes for cancer treatment.