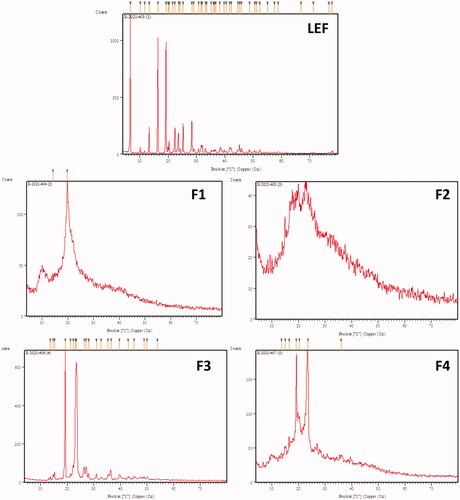
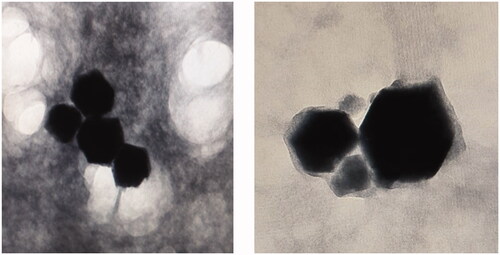
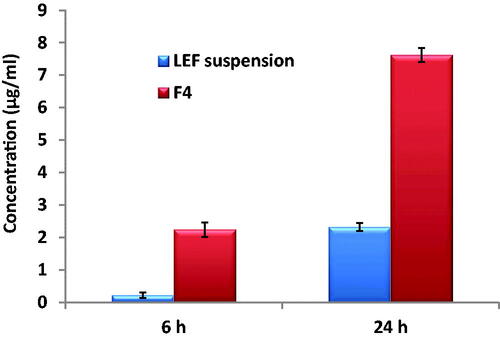
Figures & data
Table 1. Composition of LEF loaded cubosomes.
Table 2. Colloidal characteristics and % EE of different LEF loaded cubosomes.
Table 3. Release kinetics of different LEF loaded cubosomes.
Figure 1. (A) Effect of GMO concentration on particle size and PDI of cubosomes. (B) Effect of OA concentration on particle size and PDI of cubosomes.
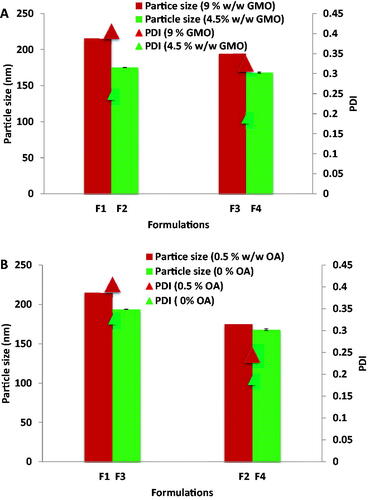
Figure 3. Cumulative percentage of LEF released from LEF loaded cubosomes using dialysis bag method.
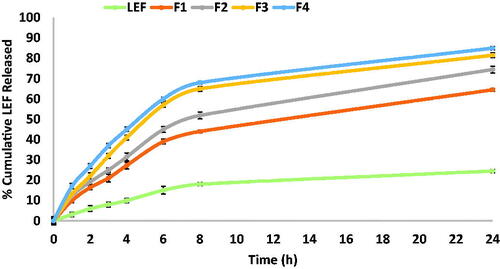
Table 4. Permeation data analysis.
Figure 4. (A) Cumulative percentage LEF permeated through skin in ex vivo permeation test. (B) Linear correlation between cumulative percentage LEF permeated in ex-vivo for LEF suspension and different cubosomal formulation within the first 8 h of the experiment.
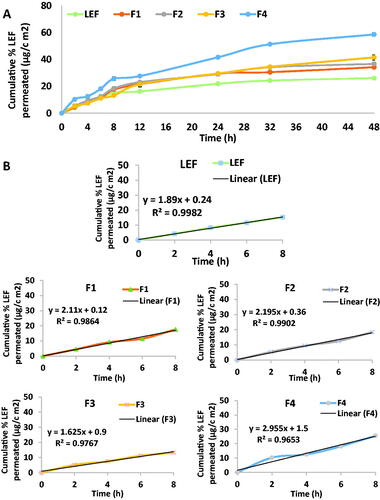
Figure 5. Correlation between percentage released and percentage permeated in different cubosomal formulations and LEF suspension.
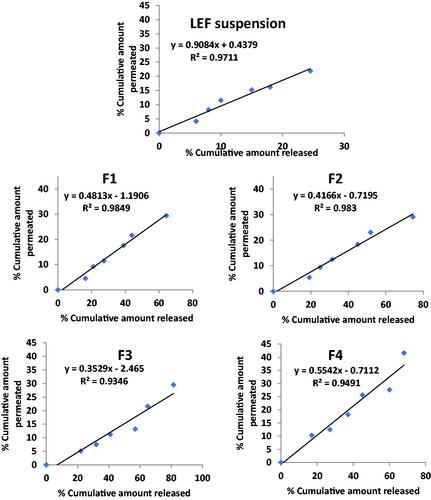
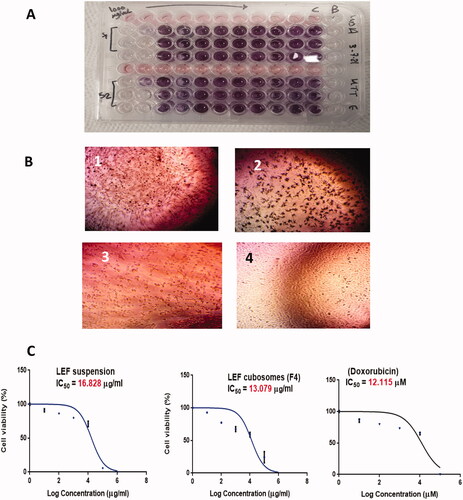
Figure 7. (A) MTT assay. (B) Morphological examination of cell line after treatment with 100 µg/mL of (1) LEF suspension, (2) LEF cubosomes (F4) and (3) Positive control (Doxorubicin). (4) MDA-MB-231 cells before treatment. (C) Percentage Cell viability of: LEF suspension, LEF cubosomes (F4) and positive control (doxorubicin).