Figures & data
Figure 1. Tumor-targeted drug delivery via EPR effect and ligand-recognition. Nanoparticles can be accumulated more in tumors than in normal tissues due to leaky tumor vasculatures and poor lymphatic drainage of tumors (EPR effect). While passive targeting is based on EPR effect, active tumor targeting is based on the selective interaction of ligand-coated nanoparticles with specific receptors overexpressed in tumor cells. The extent and intensity of EPR effect in human tumors is highly debated and active targeting is preferred for tumor-selective drug delivery.
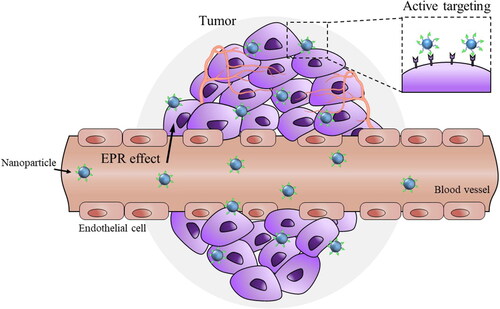
Figure 2. Strategy for active tumor-targeting via ligand-modified nanocarriers. Active targeting is achievable via surface modification of drug carriers with targeting ligands capable of interacting with antigens or receptors overexpressed (or present specifically) in tumors. Various functional ligands including folic acid, hyaluronic acid, transferrin, peptides, and antibodies, have been extensively explored to develop tumor-selective drug delivery systems.
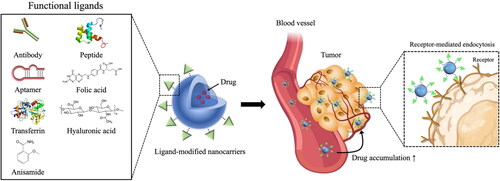
Figure 3. Strategy for enhancing intracellular uptake of anticancer drugs. Ligand-grafted nanoparticles can enhance the transcellular and paracellular transport of anticancer drugs. Surface modification of nanoparticles with cell-penetration peptides (CPPs) improves the intracellular drug uptake via multiple pathways including the energy-dependent endocytosis and direct translocation pathways. Cholesterol can be also utilized as a targeting ligand to increase the intracellular drug uptake via low-density lipoprotein (LDL) receptor-mediated endocytosis. In addition, incorporation of tight junction opening ligands to nanoparticles is applicable to enhance the paracellular drug transport.
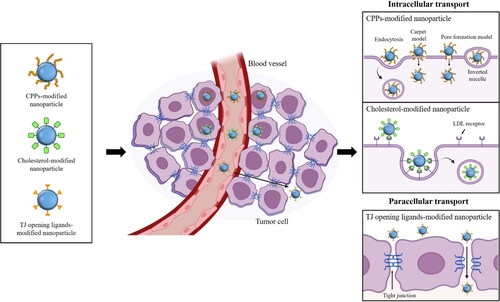
Table 1. Selected examples of CPPs based on physicochemical properties (Derakhshankhah & Jafari, Citation2018; Xie et al., Citation2020).
