Figures & data
Table 1. Immunomodulatory nano-preparations for rheumatoid arthritis.
Figure 1. Immunomodulatory nano-preparations for the delivery of self-antigens or self-antigens plus immunomodulators. (a) NPs can be used for target delivery antigens to APCs via the surface attachment of ligands. These approaches can induce antigen presentation on APCs without co-stimulatory signals, leading to T cell anergy, apoptosis, or differentiate into a regulatory phenotype. (b) Co-delivering antigens and immunomodulators via NPs can result in antigen-specific immune tolerance. Drug-loaded NPs can be phagocytosed by APCs, and then releasing drugs intracellularly. Antigen presentation will be performed under the situation of high co-inhibitory molecule levels and low co-stimulatory molecule levels, thereby resulting in T cell anergy, apoptosis, or differentiate into a regulatory phenotype. APC, antigen-presenting cell; Treg cell, regulatory T cell; PD-L1, programmed cell death 1 ligand 1; MHC, major histocompatibility complex; B7, co-stimulatory molecule CD80 and CD86.
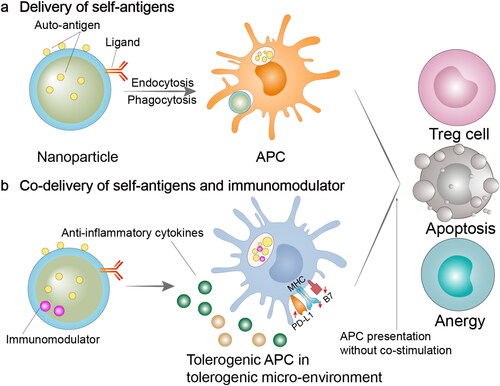
Figure 2. Schematic illustration of TPvax co-encapsulated with a multiepitope citrullinated peptide and rapamycin for anti-RA therapy by inducing antigen-specific immune tolerance. Reproduced with permission (X. Chen Xiaoyan et al., 2021a). Copyright © 2021 Elsevier Ltd.
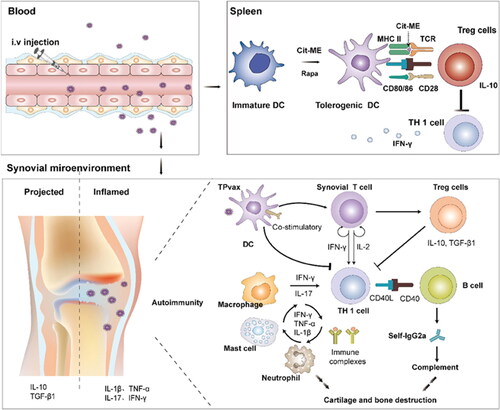
Figure 3. Tolerogenic artificial APC. In the absence of co-stimulation, artificial APCs display antigen on MHCI or MHCII to target CD8+ or CD4+ T cells, respectively. Artificial APCs have the ability to target T cells that are specific to an antigen, causing T cell anergy, apoptosis, or the generation of Tregs and Bregs. pMHC-NP, peptides coupled to major histocompatibility complex class nanoparticles; MHC, major histocompatibility complex; TCR, T-cell receptor; Tr1, Type 1 regulatory T cell; IL-10, interleukin-10.
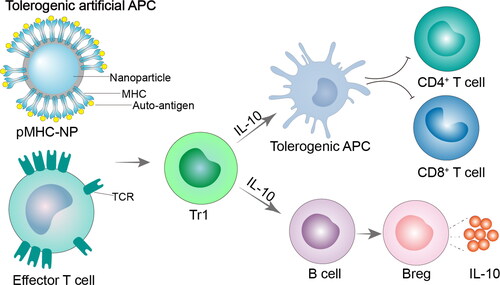
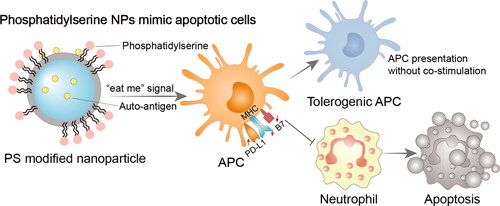
Figure 4. Immunomodulatory nano-preparations mimicking apoptotic cell avatars. Phosphatidylserine modified NPs send ‘eat me’ signal to APCs, and APCs differentiate into tolerogenic phenotypes while responding to antigen-loaded NPs. Tolerogenic APCs stop attracting neutrophils and perform immunomodulatory function. PS, phosphatidylserine; APC, antigen-presenting cell; Treg cell, regulatory T cell; PD-L1, programmed cell death 1 ligand 1; MHC, major histocompatibility complex; B7, co-stimulatory molecule CD80 and CD86.