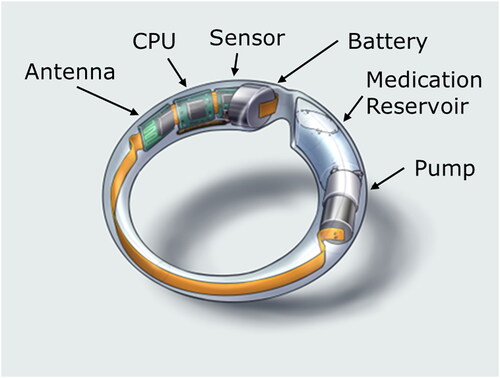
Figures & data
Table 1. Subject demographics and baseline characteristics.
Table 2. Summary of the pharmacokinetic parameters of oxybutynin and N-desethyloxybutynin after a single intravaginal dose of 3 mg oxybutynin hydrochloride using the MedRing vaginal ring device.
Figure 2. Individual pharmacokinetic profiles of oxybutynin (A) and N-desethyloxybutynin (B) up to 22 h after intravaginal dosing with 3 mg oxybutynin hydrochloride.
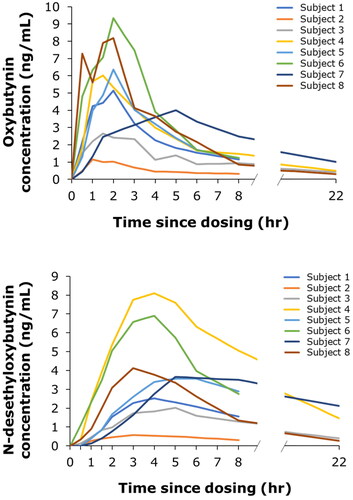
Figure 3. Mean (±SD) pharmacokinetic profiles of oxybutynin and N-desethyloxybutynin, 2 h and 6 h (A and B) after removal of MedRing and based on hormonal status (premenopause and postmenopause; C and D) after targeted intravaginal dosing with 3 mg oxybutynin hydrochloride.
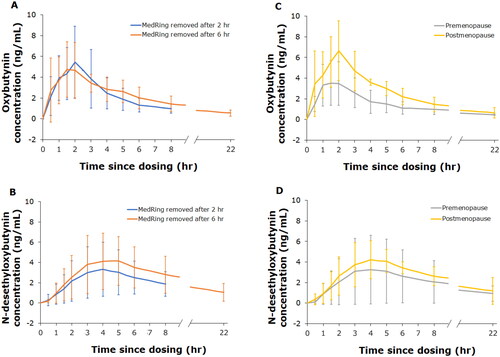
Table 3. Treatment-emergent adverse events reported during the study.
Table 4. MedRing tolerability assessments through dichotomous questions asked by the physician or the nurse during the study day.
Supplemental Material
Download Zip (271.3 KB)Data availability
The datasets generated during and/or analyzed during the study are available from the corresponding author on reasonable request.