Figures & data
Table 1 Total inhibition of HL-60 promyelocytic leukaemia cell growth by Prunus mume Sieb. et Zucc (Ume) extract. The medium (RPMI-1640) with cells, but without Ume extract was used as positive control, while DMSO solution was added as control for the Ume. Data are presented as the mean ± SD values for n = 3 separate observations
Table 2 Total inhibition of Kato-III cell growth by Prunus mume Sieb. et Zucc (Ume) extract. The medium (RPMI-1640) with cells, but without Ume extract was used as positive control, while DMSO was added as control for the Ume extract. Data are presented as the mean ± SD values for n = 3 separate observations
Figure 2 Suppressive effects of the Japanese apricot, Ume (prunus mume Siev. et Zucc) on the growth of cancer cells. HL-60 promyelocytic leukaemia cell lines and Kato-III stomach cancer cell lines were grown in culture in the presence and absence of the Ume extract at 2 μL/mL. A), growth of HL-60 cells in the presence of the vehicle; B), HL-60 cells grown in the presence of Ume extract, no viable cell was found in the test sample; C), growth of Kato-III cells in the absence of the Ume extract; D), Kato-III cells grown in the presence of Ume extract. No viable cell was found in this test sample. E), human blood cells incubated in the presence of vehicle for 24 hr; and, F) human blood cells incubated in the presence of 10 μL/mL Ume extract. There was no loss of viability or cell damage in E and F.
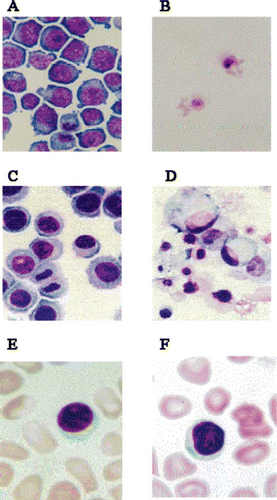
Figure 3 Silica gel chromatography column fractionated Japanese apricot juice product, Ume (Prunus mume Sieb. et Zucc). The extract derived from the condensed Ume juice killed human cancer cell lines, HL-60 and Kato-III cells shown in . This solution was subjected to Silica gel column and 5 fractions (Fra1 – Fra5) were collected, and after drying, they were dissolved in DMSO and individual fractions were tested on HL-60 promyelocytic cancer cell line. Only Fra2 showed inhibitory effects, other fractions were devoid of growth inhibition. ∗∗P < 0.01.
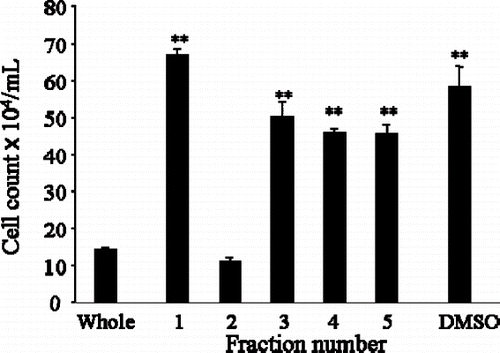
Figure 4 Suppressive effect of the Japanese apricot “Prunus mume Sieb. et Zucc” (Ume) juice product on a rapidly growing oral malignant tumour (fibrosarcoma) in a German Shepherd dog. Prior to the treatment, the animal was unable to close its mouth. The dog was given 1 g of the condensed Ume fruit juice (described in the first section of Materials and Methods) in 100 mL milk every morning during the times shown and the status of the tumour was monitored and photographed by a veterinarian doctor. At the last observation, only tumour scar was seen, and the animal was able to feed normally.
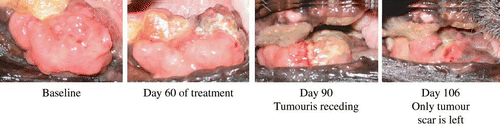
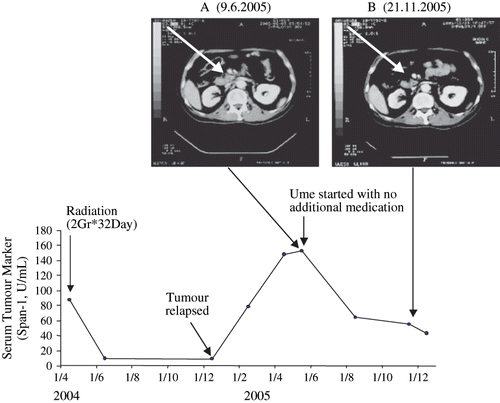
Figure 5 Part A, a CT Scan reveals the presence of a growing pancreatic tumour (at the arrow head). The CT Scan shows the tumour after a relapse following remission induced by radiation. The arrow head in part B shows the tumour has shranken to tumour scar during intake of condensed Ume fruit by the patient, 2 g/day for the entire time period shown. The lower part of the figure shows the fall of tumour marker (Span-1) during remission induced by radiation which then rises again when tumour has relapsed. The tumour marker fell again during the Ume intake and remains within the non-malignant assay range during the observation time.