Figures & data
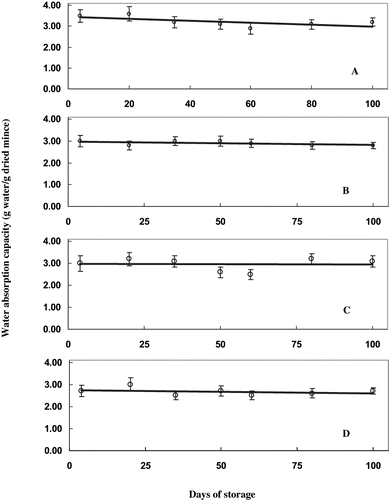
Figure 1 Ca2+ ATPase activity of proteins extracted from sardine mince treated with different concentrations of mannitol during frozen storage. Ca2+ ATPase activity is expressed as moles of inorganic phosphate released per mg of protein per minute. A) control samples, without mannitol treatment; (B) samples treated with 2% mannitol; (C) samples treated with 4% mannitol; and, (D) samples treated with 6% mannitol.
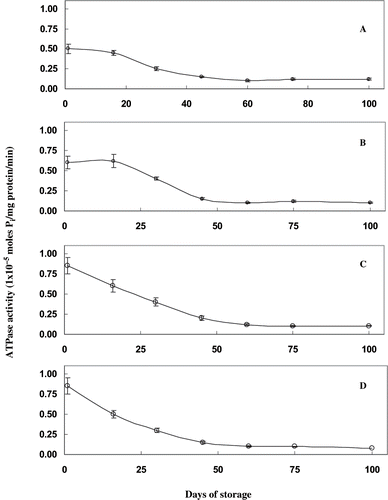
Figure 2 SDS–PAGE pattern of proteins extracted in sample buffer from sardine mince mixed with different concentrations of mannitol and frozen stored. A) immediately after freezing; and, B) at 112 days of storage. (1) standard marker proteins; (2) mince without mannitol treatment; (3) mince treated with 2% mannitol; (4) mince treated with 4% mannitol; and, (5) mince treated with 6% mannitol. MHC–Myosin Heavy Chain, MLC-Myosin Light Chain.
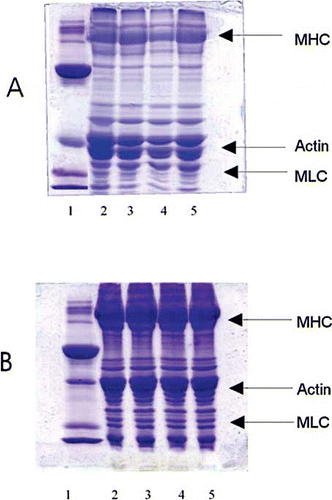
Figure 3 Thermal denaturation profile of fish proteins during heating from 25 – 95°C at pH 7.5. A). The absorbance at 287 nm was monitored during heating in thermal cuvettes with a heating rate of 1°C per minute B) first derivative plot of absorbance vs temperature.
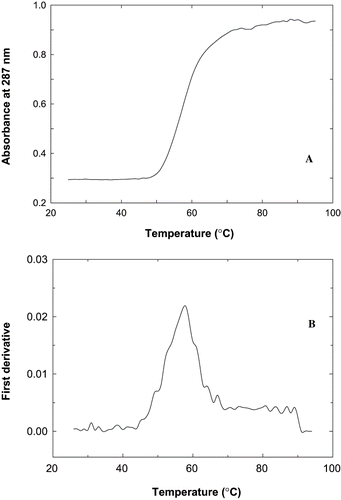
Table 1 Apparent Tm (°C) of proteins from sardine mince treated with different concentrations of mannitol during frozen storage
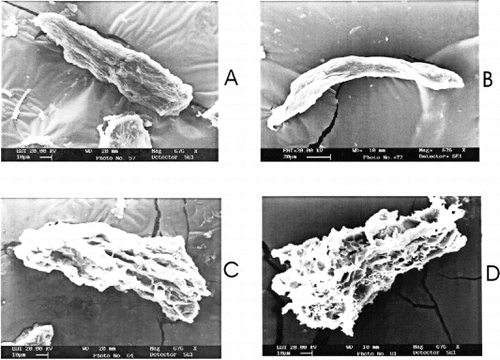
Figure 4 Scanning electron micrographs of sardine mince during frozen storage in presence of mannitol. A) control samples, without mannitol treatment immediately after freezing; B) at 120 days of storage; C) sardine mince treated with 6% mannitol immediately after freezing; and, D) at 120 days of storage.