Figures & data
Table 1 Anhydrous glass transition temperature (Tg) as well as glass transition temperature (Tg´), and onset of ice melting temperature (Tm´) of maximally freeze-concentrated solutions of lactose/sucrose and lactose/sucrose protein and lactose/sucrose cornstarch mixtures (Cg´) for various sugar/proteins and polysaccharide mixtures. A 15 min annealing time was used for all solutions
Figure 1 Thermograms of 20%, lactose/ sucrose, lactose/ sucrose/ albumin (1:1), lactose/ sucrose/ gelatin (1:1:1), lactose/ sucrose/ cornstarch (1:1:1), and lactose/ sucrose/ gelatin/c ornstarch (1:1:1:1) solutions isothermally annealed at (Tm´−1)°C for 15 minutes before determination of the transitions during rewarming from –100 to 20°C at 5°C min−1.
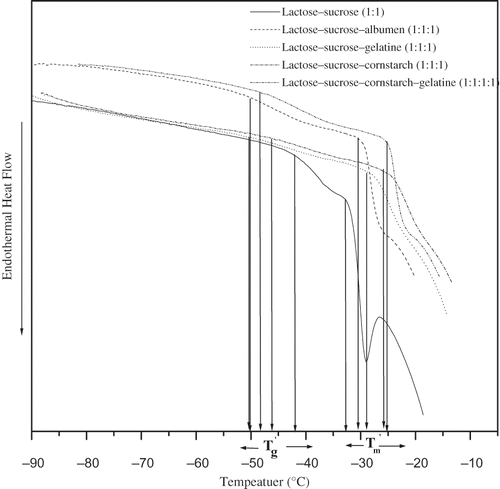
Figure 2 DSC thermograms (Second scan) of freeze-dried anhydrous, lactose/ sucrose (1:1), lactose/ sucrose/ albumin (1:1:1), lactose/ sucrose/ gelatin (1:1:1), lactose/ ucrose/ cornstarch (1:1:1), and lactose/ sucrose/ gelatin/ cornstarch (1:1:1:1). The arrow indicates the onset of Tg.
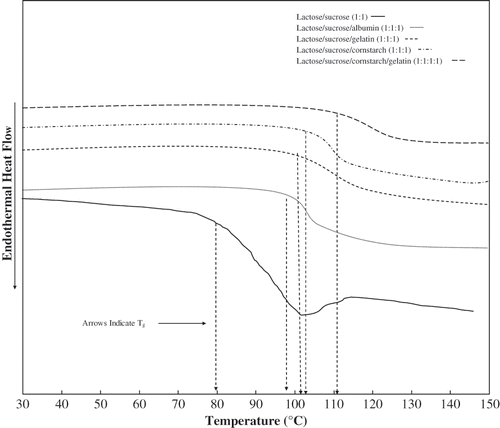
Figure 3 State diagrams for lactose/sucrose, lactose/sucrose/albumin (1:1:1), lactose/ sucrose/ gelatin (1:1:1), lactose/ sucrose/ cornstarch (1:1:1), and lactose/ sucrose/ cornstarch/ gelatin (1:1:1:1). Tg curve was predicted by the Gordon and Taylor equation (Gordon and Taylor, 1952).[Citation37] Below Tg all solutions were in the glassy state. Maximally freeze concentrated solutions showed constant Tg´ (onset of glass transition temperature) and Tm´ (onset of ice melting temperature) values. All solution showed maximum concentration of solute in the unfrozen phase at Cg´.
![Figure 3 State diagrams for lactose/sucrose, lactose/sucrose/albumin (1:1:1), lactose/ sucrose/ gelatin (1:1:1), lactose/ sucrose/ cornstarch (1:1:1), and lactose/ sucrose/ cornstarch/ gelatin (1:1:1:1). Tg curve was predicted by the Gordon and Taylor equation (Gordon and Taylor, 1952).[Citation37] Below Tg all solutions were in the glassy state. Maximally freeze concentrated solutions showed constant Tg´ (onset of glass transition temperature) and Tm´ (onset of ice melting temperature) values. All solution showed maximum concentration of solute in the unfrozen phase at Cg´.](/cms/asset/8a13ece6-95aa-4f90-b8df-8d67a8c08ba1/ljfp_a_326085_o_f0003g.gif)
Table 2 The glass transition temperature (Tg´), and onset of ice melting temperature (Tm´) of maximally freeze-concentrated solutions of sugar mixtures/proteins/cornstarch mixtures. A 15 min annealing time was used for all solutions
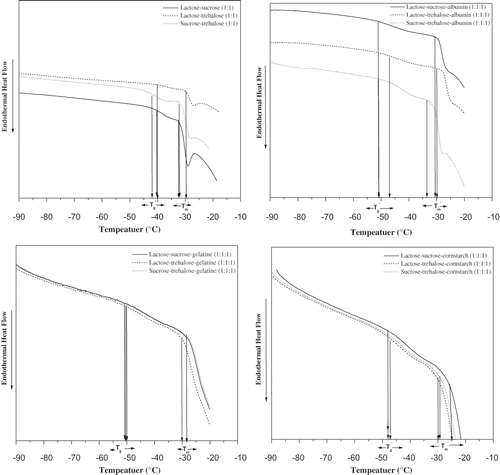
Figure 4 a) Thermograms of 20% solutions, lactose/sucrose (1:1), lactose/ trehalose (1:1), and sucrose/ trehalose isothermally annealed at (Tm´−1) °C for 15 minutes before determination of the transitions during rewarming from –100 to 20°C at 5°C min−1. b) Thermograms of 20% solutions, lactose/ sucrose/ albumin (1:1:1), lactose/ trehalose/ albumin (1:1:1), and sucrose/ trehalose/ albumin (1:1:1) isothermally annealed at (Tm´−1) °C for 15 minutes before determination of the transitions during rewarming from –100 to 20°C at 5°C min−1. c) Thermograms of 20% solutions, lactose/ sucrose/ gelatin (1:1:1), lactose/ trehalose/ gelatin (1:1:1), and sucrose/trehalose/gelatin (1:1:1) isothermally annealed at (Tm´−1) °C for 15 minutes before determination of the transitions during rewarming from –100 to 20°C at 5°C min−1. d) Thermograms of 20%, lactose/sucrose/cornstarch (1:1:1), lactose/ trehalose /cornstarch (1:1:1), and sucrose/ trehalose/ cornstarch (1:1:1) isothermally annealed at Tm´−1 for 15 minutes before determination of the transitions during rewarming from –100 to 20°C at 5°C min−1.