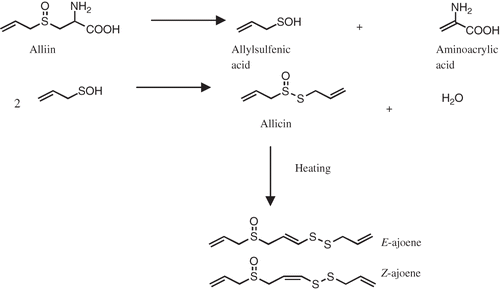
Figures & data
Figure 2 Scavenging activity of ajoene toward DPPH radical. The standard reaction mixture contained 500μM DPPH in ethanol. Ajoene was added to the final concentrations indicated on the axis. The results represent the mean values ± SD from three independent experiments.
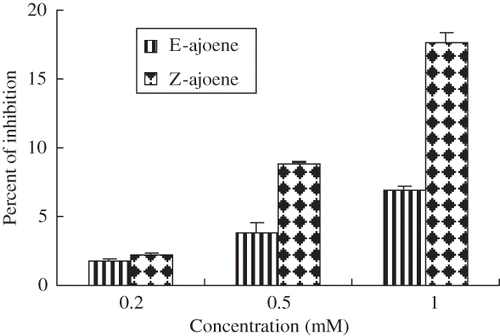
Table 1 Antioxidant activities of E- and Z-ajoene and known antioxidant α-tocopherol
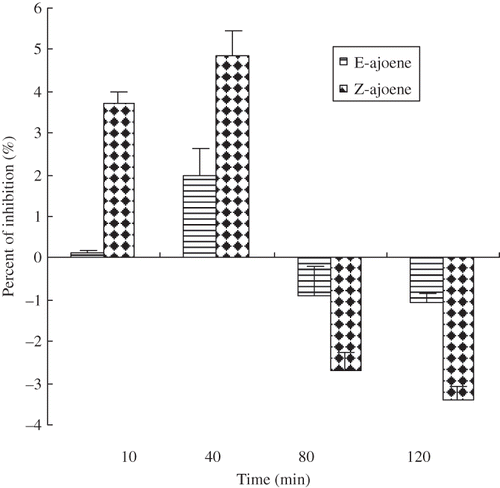
Figure 3 Scavenging activities of ajoene toward hydroxyl radical. The standard reaction mixture contained, at final concentrations, 10 mM sodium phosphate (pH 7.4), 2.5 mM 2-deoxyribose, 1.0 mM iron ammonium sulfate, 1.04 mM EDTA, 1.0 mM ascorbic acid, 0.1 M H2O2, 2.8% TCA% and 1% TBA. Ajoene were added to the final concentrations indicated on the axis. The results represent the mean values ± SD from three independent experiments. Error bars indicate standard deviations; where no error bars are visible, the standard deviation is less than the size of the symbol.
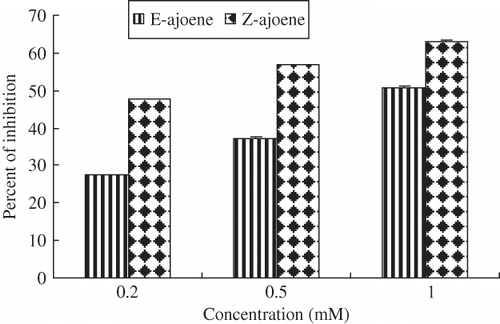
Figure 4 Scavenging activities of ajoene toward superoxide anion. The standard reaction mixture contained, at final concentrations, NADH (117 μM), NBT (37.5 μM), and phenazine methosulfate (PMS, 15 μM) in sodium phosphate (0.1 M, pH 7.4). The test compounds or standard antioxidant (1mM) were added and the reaction was started by the addition of PMS. The results represent the mean values ± SD from three independent experiments. Error bars indicate standard deviations.