Figures & data
Figure 1 Adsorption isotherms for M100 and M180 maltodextrins, sodium ascorbate, and blends of M100 and M180 with ascorbate at 22°C. Data points are connected by trend lines. Formulations are shown by: ![]()
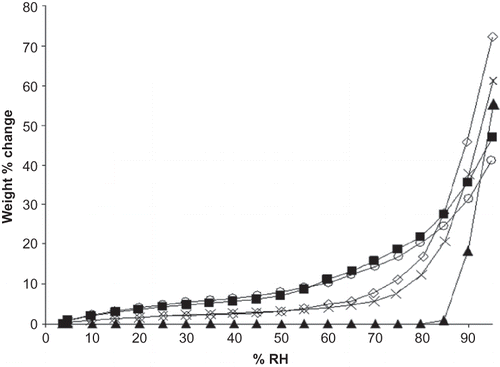
Figure 2 Glass transition temperature (Tg) for maltodextrins equilibrated at increasing RH analyzed by DSC. Tg values were determined from the onset temperature. Data points are connected by trend lines. Selected maltodextrins are shown by:![]()
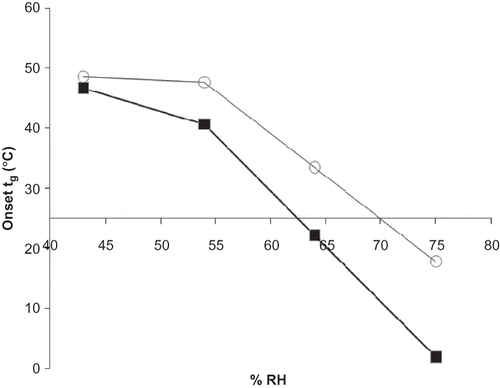
Figure 3 Difference in percent moisture sorption between experimental and predicted moisture sorption isotherms of M100 maltodextrin+ascorbate and M180 maltodextrin+ascorbate blends exposed to 0–95% RH and 25C. Predicted isotherms were calculated from the experimental moisture sorption isotherms of the individual ingredients. The predicted moisture sorption isotherms of the mixtures were then compared to those obtained experimentally for the same mixture and the difference was plotted against RH. Data points are connected by trend lines. Different formulations are shown by: ![]()
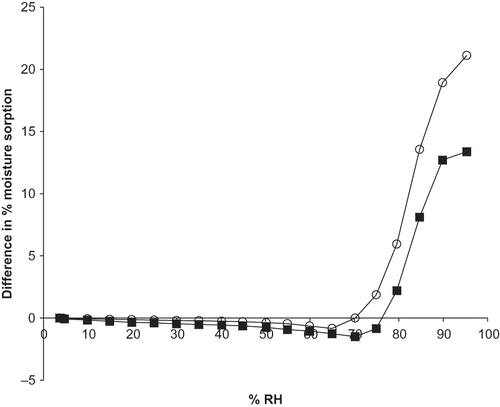
Figure 4 Sodium ascorbate stability at different storage RHs, formulated with and without maltodextrin (M100). A) 98% RH; B) 85% RH; C) 75% RH; D) 43% RH. Data points are connected by trend lines. Individual ascorbate and M100 maltodextrin mixtures are shown by: ![]()
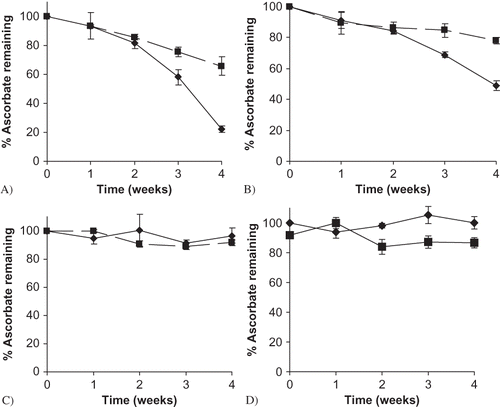
Table 1 Endpoint vitamin C degradation after 4 weeks of storage at select RHs for individual sodium ascorbate and binary mixtures of sodium ascorbate and M100 or M180 maltodextrin. Results are reported as % degradation with standard deviations, and different letters indicate significant differences.Footnote a
Figure 5 Weight change (%) versus % sodium ascorbate remaining for individual sodium ascorbate and its blend with M100 maltodextrin stored at 98% RH and 25°C up to 4 weeks. Data points are connected by trend lines. Trends for ascorbate and its blend are show by: ![]()
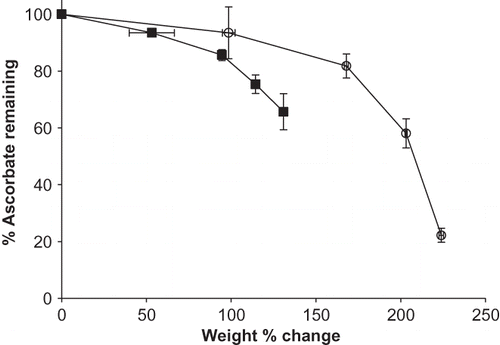
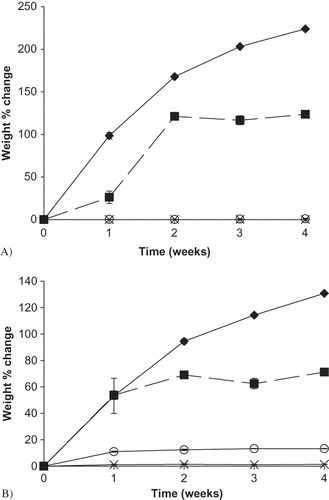
Figure 6 Weight change (%) over time for sodium ascorbate and its blends with M100 and M180 maltdoextrin stored up to 4 weeks at 22°C and select RH conditions. A) Weight change (%) over time for individual sodium ascorbate samples during controlled RH storage. Data points are connected by trend lines. Ascorbate samples at each RH are shown by: ![]()