Figures & data
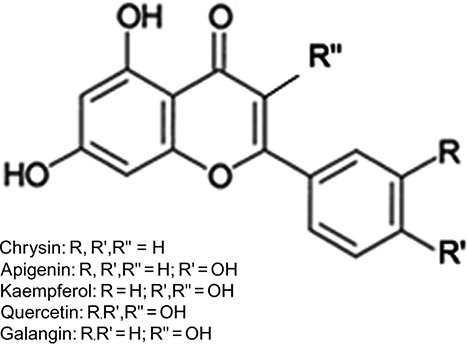
Figure 1 Typical HPLC chromatogram of honey flavonoids. EtE obtained as described under Materials and Methods was dissolved in DMSO, diluted in methanol, and analyzed by HPLC/MS. Peaks: luteolin (A), quercetin (B), 8-methoxykaempferol (C), apigenin (E), kaempferol (F), isorhamnetin (G), acacetin (J), tamarixetin (K), chrysin (N), and galangin (O). Peaks D, H, I, L, and M are unidentified compounds.
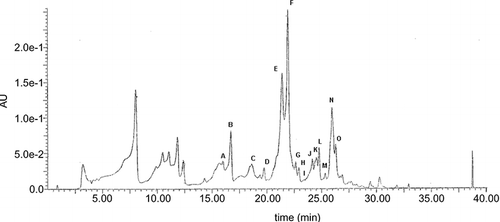
Table 1 In vitro antimicrobial activity of different concentrations of honey EtE tested against C. albicans using agar-well diffusion and broth microdilution methods
Table 2 Mean viable counts of C. albican s treated with honey EtE
Figure 2 Fluorescence micrographs of Candida albicans cells showing their viability after 24-h incubation in YNB, in the absence (a) or in the presence (b) of EtE. (c) negative control.

Figure 3 Typical HPLC chromatogram for flavonoid uptake by Candida albicans cells. Flavonoids were extracted from either the whole cell suspension lysate (—) or cell pellet lysate (- - -) after 6-h incubation in the presence of EtE. Apigenin (E), kaempferol (F), chrysin (N), galangin (O).
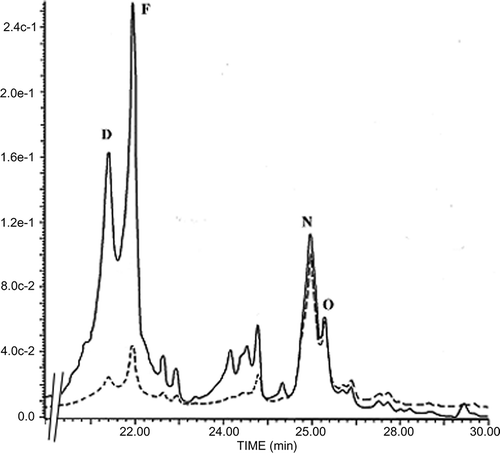
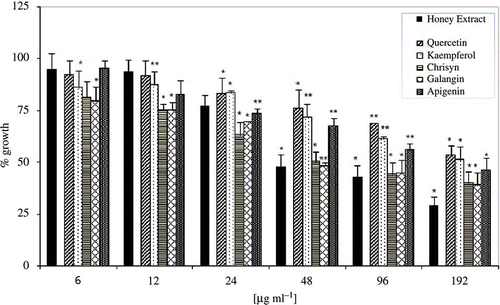
Figure 5 Antimicrobial activities of EtE (black bar) and commercial pure phenolic compounds. The data are expressed assuming 100% growth for untreated control. Bars represent the average for four separate experiments and the error bars show the standard deviation. The distinction between the average values obtained was statistically significant at P < 0.05 (*) or P < 0.001 (**) according to Student's t test.