Figures & data
Figure 1 (a) Calculated increase of pressure inside the measurement cell as filling volume increases for a temperature increase of ΔT 20–130°C. (b) Temperature profile for UHT-Milk measured inside the treatment chamber (fixed silicon oil temperature of 140°C) (color figure available online).
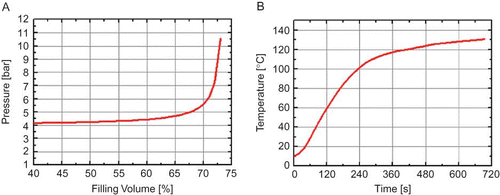
Figure 2 (a) Measured slope shift in a certified temperature-stable standard buffer solution versus 99.7% of Nernst-slope up to 100°C[20], data up to 130°C were extrapolated. (b) Linear function of the electrode response from 20°C to 120°C in correlation with pH (color figure available online).
![Figure 2 (a) Measured slope shift in a certified temperature-stable standard buffer solution versus 99.7% of Nernst-slope up to 100°C[20], data up to 130°C were extrapolated. (b) Linear function of the electrode response from 20°C to 120°C in correlation with pH (color figure available online).](/cms/asset/5ecf635d-e6fd-4681-8fc8-4a05cd86a893/ljfp_a_446058_o_f0002g.jpg)
Figure 3 (a) pH shift in PBS buffer solution (0.05 M) and certified reference buffer-solution; (b) pH shift in ACES- (0.05 M) and TRIS buffer solution (0.05 M); C) pH shift in acetate- (0.05 M) and citrate buffer solution (0.05 M). Error bars indicate the standard error (color figure available online).
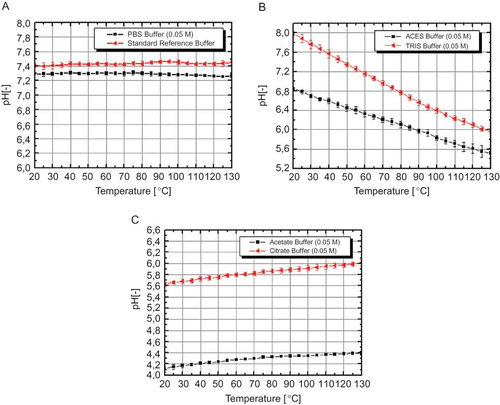
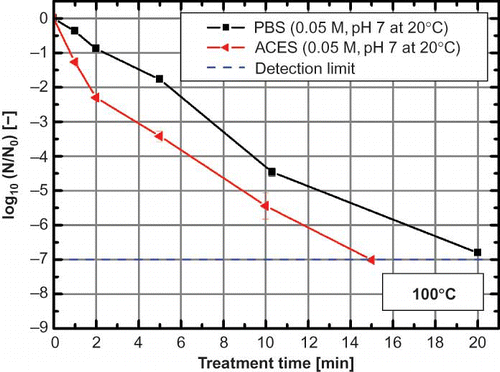
Figure 4 Thermal inactivation of Bacillus subtilis spores at 100°C in PBS and ACES buffer with an initial pH of 7 (0.05 M) at 20°C. Error bars indicate the standard error (color figure available online).
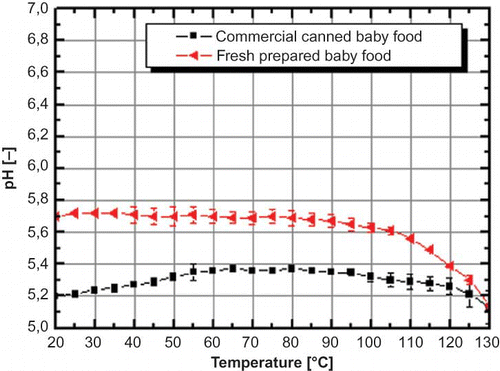
![Figure 5 (a) pH shift in raw milk and ultra heat treated milk (UHT-Milk) as well as; (b) pH shift in raw milk and its components (whey protein, casein and lactose [4.5%]) in dependence of temperature. Error bars indicate the standard error (color figure available online).](/cms/asset/1eec8a0f-8ed9-4bc7-ae94-72d395bdbc1b/ljfp_a_446058_o_f0005g.jpg)