Figures & data
Figure 1 Schematic diagram of the interaction of a crystalline solid, such as sucrose, with water vapor in the atmosphere at various relative humility values, where RHE = environmental relative humidity and RHo = critical relative humidity. Stages A through D are explained in the text (color figure available online).
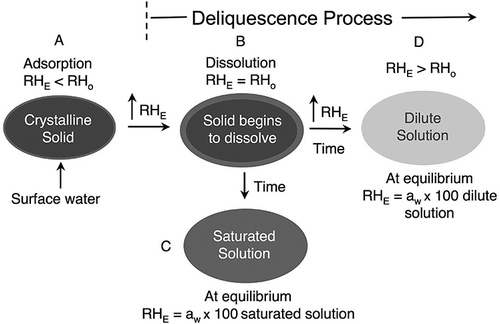
Figure 2 Gibbs free energy of a solid and its aqueous solution as a function of %RH. The %RH at which the solid and the solution Gibbs free energies become equal is the deliquescence point (DRH or RHo ) (excerpted from reference 2).
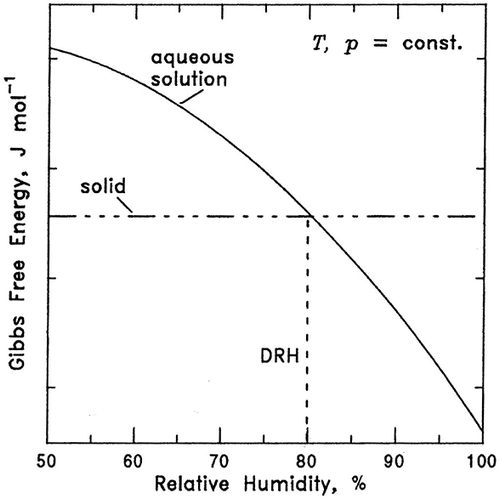
Table 1 Saturation solubility data for sucrose in water in both g sucrose per g water and %MC (dry basis) units as a function of temperature from Bubník and Kadlec.[Citation28]
Figure 3 Crystalline sucrose DDIs obtained using a 300 mL/min flow rate and saturated sucrose solution values (moisture content values from and aw values from this study []) at 15, 25, and 35°C.
![Figure 3 Crystalline sucrose DDIs obtained using a 300 mL/min flow rate and saturated sucrose solution values (moisture content values from Table 1 and aw values from this study [Table 2]) at 15, 25, and 35°C.](/cms/asset/61f5d77d-4e07-4f40-9f7a-6ec9f1ec2f84/ljfp_a_447814_o_f0003g.gif)