Figures & data
Figure 1 Effect of cosolvents on α-amylase activity at different pH. The curves represent the α-amylase activity of (∀) control, and in presence 40% (w/v) (8) glycerol, (X) sorbitol, (–) trehalose, and (M) sucrose.
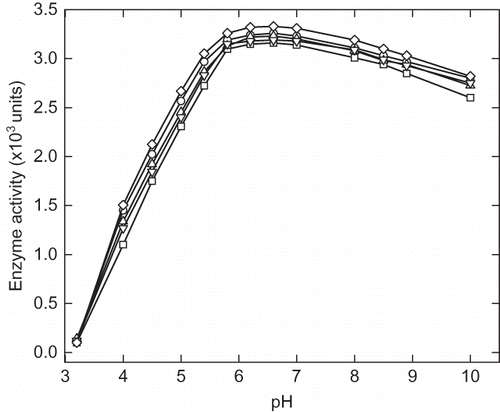
Figure 2 pH induced aggregation of α-amylase. The protein aggregation was monitored by recording the absorbance at 360 nm at each pH ranging from 1.5 to 7.
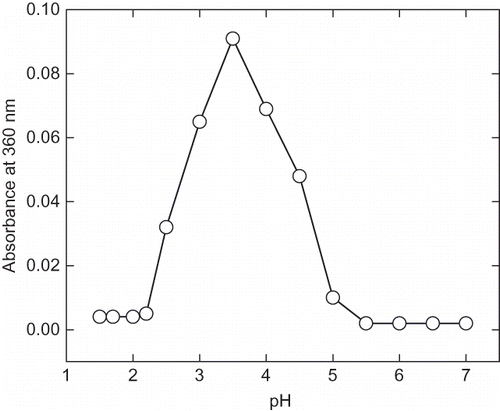
Figure 3 Intrinsic fluorescence spectra of α-amylase at different pH ranging from pH 1.5 to 7. The enzyme samples were incubated at different pH for 12 h before recording the spectra. The fluorescence spectra are represented as: (a) pH 7, (b) pH 6, (c) pH 5, (d) pH 4, (e) pH 3, (f) pH 2.75, (g) pH 2.25, and (h) pH 1.5.
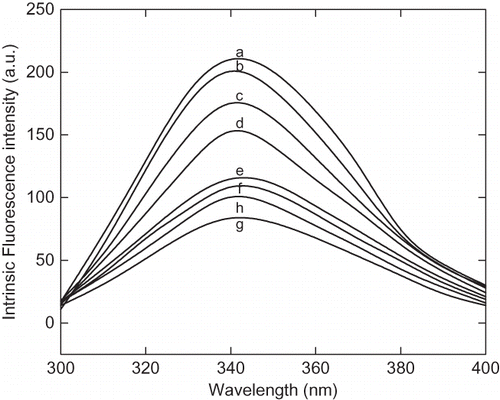
Figure 4a The 8-anilinonaphathalene-1-sulphonic acid (ANS) fluorescence spectra of α-amylase at different pH ranging from pH 1.5 to 7. The curves are represented as: (a) pH 7, 6.5 and 6.00, (b) pH 4.5, (c) pH 4.00, (d) pH 3.5, (e) pH 3.00, (f) pH 2.5, (g) pH 2.25, (h) pH 2.00, (i) pH 1.75, and (j) pH 1.5.
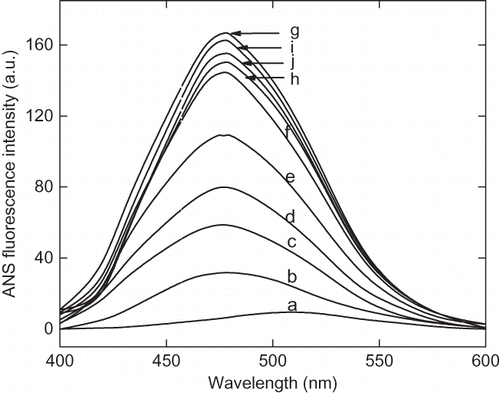
Figure 4b Variation of 8-anilinonaphathalene-1-sulphonic acid (ANS) fluorescence intensity of α-amylase with change in pH at 25°C. After incubating the enzyme solution at different pH the aliquots ANS stock solution was added, mixed and stand for 20 min in dark. The excitation wavelength was fixed at 380 nm and emission spectra were collected between 400–600 nm.
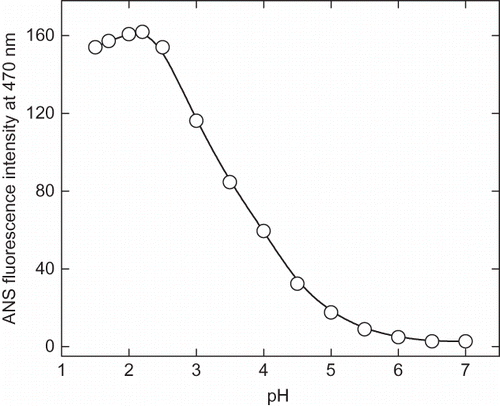
Figure 4c Change in 8-anilinonaphathalene-1-sulphonic acid (ANS) emission maximum as a function of pH. The emission spectra were collected between 400–600 nm and excitation wavelength was fixed at 380 nm.
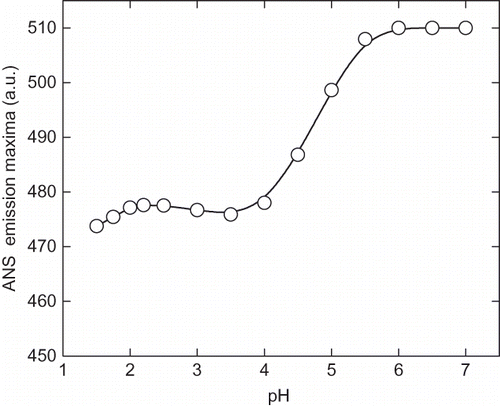
Figure 5 Stern-Volmer plot of acrylamide quenching of intrinsic fluorescence of enzyme at pH (a) 7.0, (b) 2.25, and (c) 1.5. Aliquot of 2M stock solutions of acrylamide added sequentially and excitation wavelength was fixed at 280 nm and emission maxima were recorded at 340 nm. The F0 and F are the fluorescence intensity of the enzyme in absence and presence of acrylamide, respectively. The ratio of F0 and F indicates the relative quenching of intrinsic fluorescence under given condition.
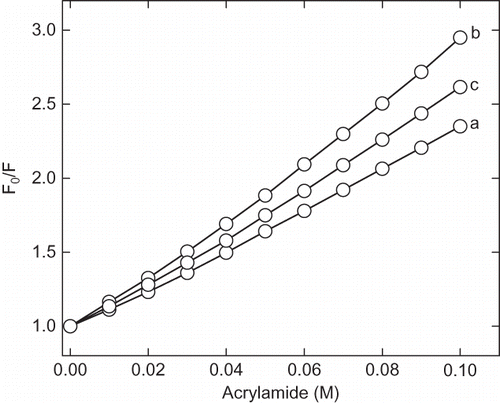
Figure 6 The 8-anilinonaphathalene-1-sulphonic acid (ANS) fluorescence spectra of enzyme at native and acidic pH in presence of cosolvents. Enzyme solutions were incubated at acidic pH in presence of cosolvents for 12 h before recording the spectra. The curves (a) and (b) represent pH 7 and 2.25 in absence of cosolvents, respectively. Curves (c), (d), (e), and (f) represent the spectra in presence of 30% of trehalose, sorbitol, sucrose, and glycerol at pH 2.25, respectively. The curve (g) and represent 100 mM CaCl2 and 250 mM of NaCl, respectively.
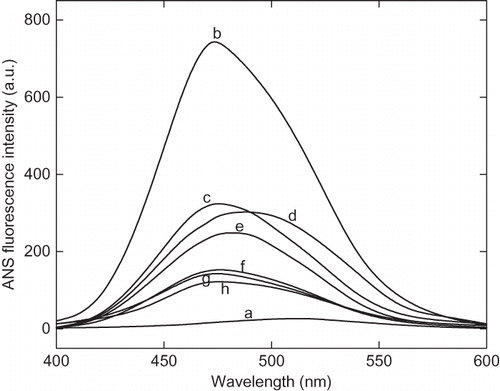
Figure 7 (a) Near UV-circular dichroic spectra of enzyme at pH (1) 7.0 and (2) 2.25. The enzyme solutions were incubated for 12 h before the circular dichroism measurement. The protein concentrations were maintained to be 1 mg/mL. The [θ]MRW is molar residual elipticity of enzyme. (b) Far UV-circular dichroic spectra of α-amylase at pH (1) 7.0 and (2) 2.25. The enzyme solutions were incubated for 12 h before the CD measurement. The protein concentrations were maintained to be 0.26 mg/mL. The [θ]MRW is molar residual ellipticity of enzyme.
![Figure 7 (a) Near UV-circular dichroic spectra of enzyme at pH (1) 7.0 and (2) 2.25. The enzyme solutions were incubated for 12 h before the circular dichroism measurement. The protein concentrations were maintained to be 1 mg/mL. The [θ]MRW is molar residual elipticity of enzyme. (b) Far UV-circular dichroic spectra of α-amylase at pH (1) 7.0 and (2) 2.25. The enzyme solutions were incubated for 12 h before the CD measurement. The protein concentrations were maintained to be 0.26 mg/mL. The [θ]MRW is molar residual ellipticity of enzyme.](/cms/asset/2c19350c-69de-447d-ac96-9e9d641db17d/ljfp_a_459788_o_f0009g.gif)