Figures & data
Figure 1 Moisture sorption isotherms for single and binary mixtures of vitamin C forms. A. Sorption isotherms for single vitamins forms. Isotherms for each ingredient are shown by: ![]()
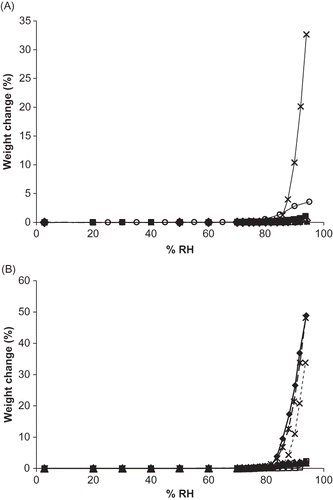
Figure 2 Moisture sorption isotherms for a single, binary, ternary, and quaternary mixture containing sodium ascorbate. Individual ingredients are abbreviated as: A = ascorbic acid, N = sodium ascorbate, C = calcium ascorbate, P = ascorbyl palmitate. Isotherms for each mixture are shown by: ![]()
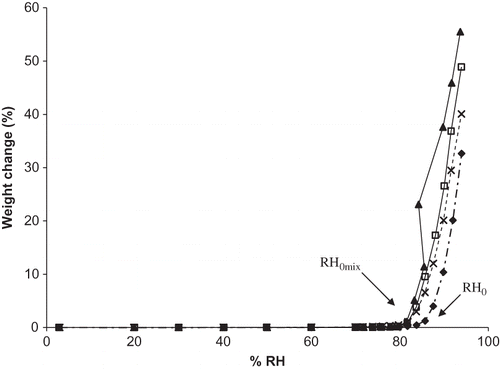
Table 1 Water activity, pH, deliquescence point, and solubility information for ingredients and their mixtures at 25°C
Figure 3 Moisture sorption isotherms for individual DHAA and sodium ascorbate and binary mixtures of the two in ratios of 50:50, 25:75, and 10:90 DHAA:N. Individual ingredients are abbreviated as: N = sodium ascorbate, D = dehydroascorbic acid. Isotherms for individual ingredients and mixtures are shown by: ![]()
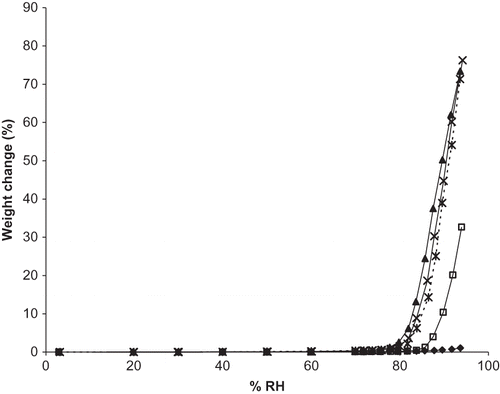
Figure 4 Weight gain (%) for individual vitamin C forms and their binary mixtures during storage up to 12 weeks at 98% RH. A. Weight gain (%) for individual vitamin C forms over time. Individual ingredients are abbreviated as: A = ascorbic acid, N = sodium ascorbate, C = calcium ascorbate, P = ascorbyl palmitate. Percent weight gain for individual ingredients is shown by: ![]()
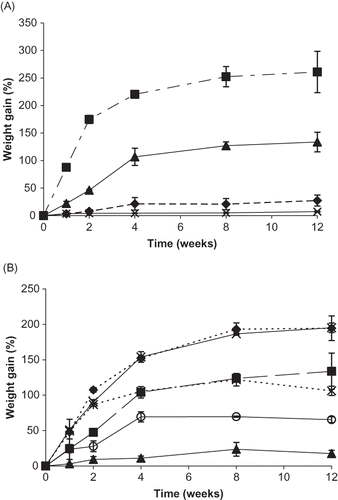
Figure 5 Experimental and predicted moisture sorption isotherms for a ternary mixture of vitamin C forms (ANC). Individual ingredients are abbreviated as: A = ascorbic acid, N = sodium ascorbate, C = calcium ascorbate. Sorption isotherms are shown by: ![]()
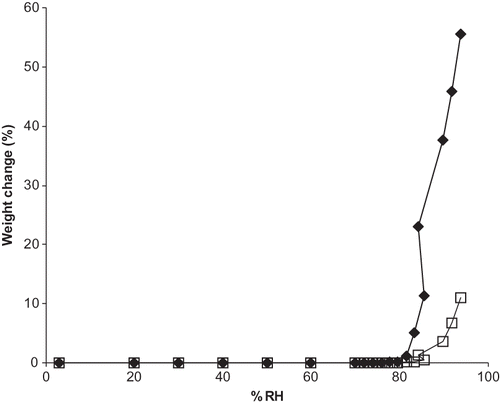
Figure 6 Weight gain (%) for individual DHAA and binary mixtures of DHAA and sodium ascorbate in ratios of 50:50, 25:75, and 10:90 DHAA:N over time during storage at 98% RH. Individual ingredients are abbreviated as: N = sodium ascorbate, D = dehydroascorbic acid. Percent weight gain for mixtures is shown by: ![]()
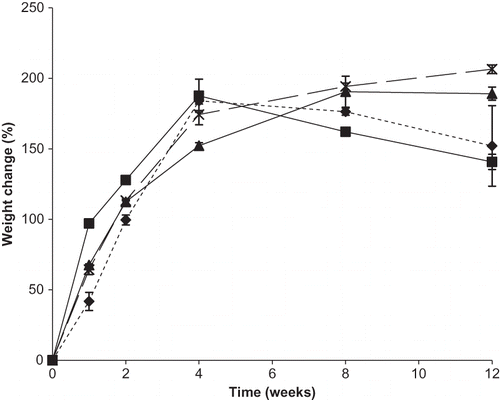
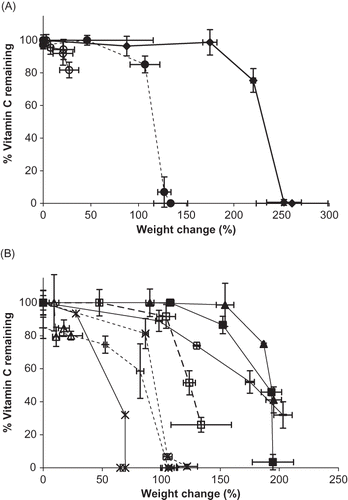
Figure 7 Vitamin C stability for individual vitamin forms and their binary mixtures during storage up to 12 weeks at 98% RH. A. Percent vitamin C remaining over time for different vitamin forms. Individual ingredients are abbreviated as: A = ascorbic acid, N = sodium ascorbate, C = calcium ascorbate. Percent vitamin C remaining for individual vitamins is shown by: ![]()
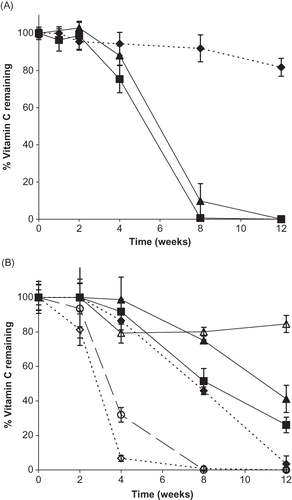
Table 2 Endpoint vitamin C degradation after 12 weeks of storage at select RHs for individual vitamin C forms and in vitamin C ingredient mixtures. Results are reported as % degradation with standard deviations and different letters indicate significant differencesa. Each formulation was stored at three RHs selected to be above, near, and below the deliquescence point of the blend
Figure 8 Percent of reduced ascorbate remaining over time for binary mixtures of dehydroascorbic acid and sodium ascorbate in ratios of 50:50, 25:75, or 10:90 DHAA:N stored at 98% RH. Individual ingredients are abbreviated as: N = sodium ascorbate, D = dehydroascorbic acid. Percent reduced ascorbate remaining in mixtures is shown by: ![]()
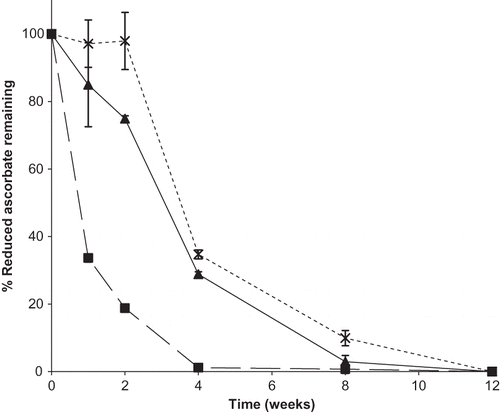
Figure 9 Percent weight gain versus % vitamin C remaining for vitamin C forms and their blends stored at 98% RH and 25°C up to 12 weeks. A. Percent weight gain versus % vitamin C remaining for individual vitamin C forms. Individual ingredients are abbreviated as: A = ascorbic acid, N = sodium ascorbate, C = calcium ascorbate. Trends for individual vitamins are shown by: ![]()