Figures & data
Figure 1 ACE-inhibitory activity and DH of casein hydrolysates prepared under casein concentration 10% (w/v), original pH 8.5, reaction temperature 55°C, E/S ratio 1 kU/g proteins with different hydrolysis time. The final concentration of casein or casein hydrolysates for ACE-inhibitory activity assay was 50 μg/mL on protein or peptide basis. Column chart was for ACE-inhibitory activity, and graph chart was for DH.
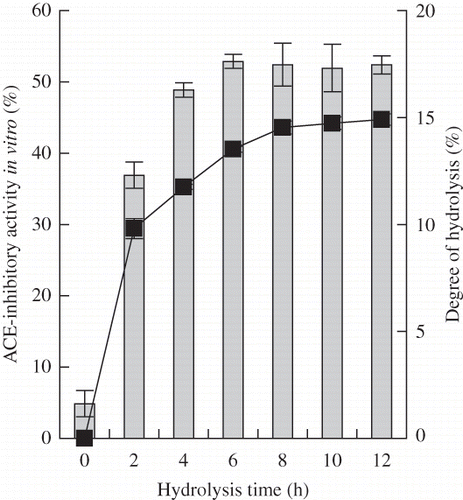
Figure 2 Effect of substrate concentration (%, by weight) on the varying amount of free amino groups of the modified casein hydrolysates during plastein reaction. The reaction was carried out at original pH of 6.8, E/S ratio of 3 kU/g peptides, reaction temperature of 35°C, and reaction time of 5 h. (Color figure available online.)
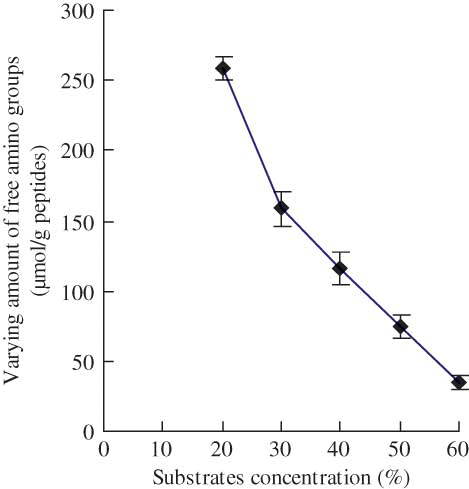
Figure 3 Effect of E/S ratio on the varying amount of free amino groups of the modified casein hydrolysates during plastein reaction. The reaction was carried out at original pH of 6.8, substrate concentration of 40% by weight, reaction temperature of 35°C, and reaction time of 5 h. (Color figure available online.)
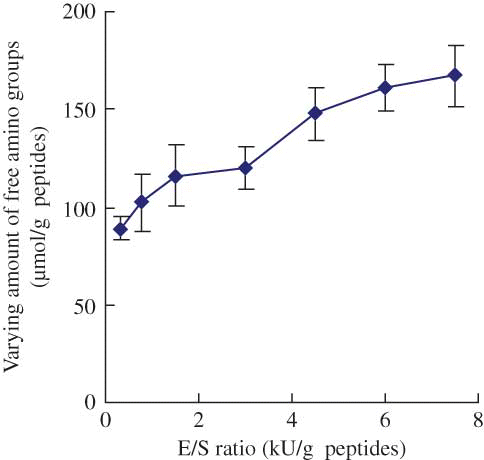
Figure 4 Effect of reaction temperature on the varying amount of free amino groups of the modified casein hydrolysates during plastein reaction. The reaction was carried out at original pH of 6.8, substrate concentration of 40% by weight, E/S ratio of 3 kU·g−1 peptides, and reaction time of 5 h. (Color figure available online.)
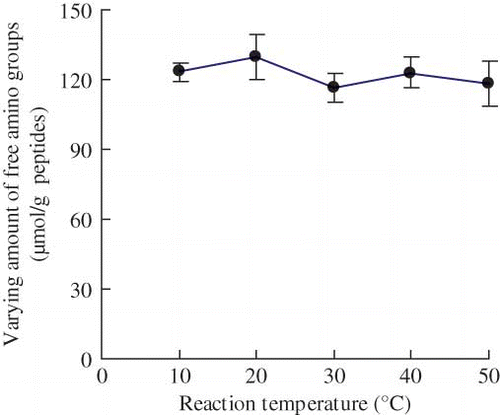
Table 1 Varying amount of free amino groups and ACE-inhibitory activities of six modified casein hydrolysates prepared
Table 2 Impacts of further hydrolysis of casein hydrolysates by Neutrase on ACE-inhibitory activity in vitro of the hydrolyzed casein hydrolysates obtained
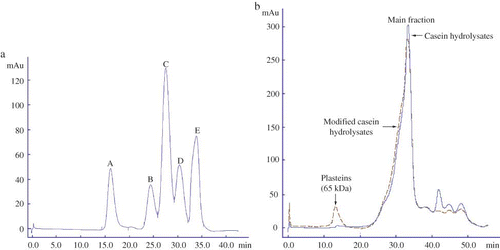
Figure 5 Analysis of the standards (a) casein hydrolysates and the modified hydrolysates (b) in AKTA Explorer 100 with Superdex-75 column. The analysis was carried out at a flow rate of 0.5 mL/min with 0.1 mol/L Na2HPO4 and NaOH buffer (pH 12) and monitored at 280 nm. The standards were bovine serum albumin (66.2 kDa), cytochrome c (12.4 kDa), insulin (5.7 kDa), oxidized L-glutathione (0.6 kDa), and L-tyrosine (0.2 kDa), and appeared as peak A to E in (a), respectively. Casein hydrolysates were prepared by hydrolyzing casein under casein concentration 10% (w/v), original pH 8.5, reaction temperature 55°C, E/S ratio 1 kU/g proteins, and reaction time 6 h to a DH of 13.5%. The modified hydrolysates were prepared from casein hydrolysates by Nutrase-catalyzed plastein reaction at an original pH of 6.8, substrate concentration of 40% (by weight), Neutrase addition level of 3 kU/g peptides, reaction temperature of 35°C, and reaction time of 6 h. (Color figure available online.)