Figures & data
Table 1 Composition of model systems and heating conditions
Figure 1 HPLC chromatogram of egg white protein (a) prior to enzymatic hydrolysis, (b) after 2 h of pepsin hydrolysis, 1–5 egg white protein fractions.
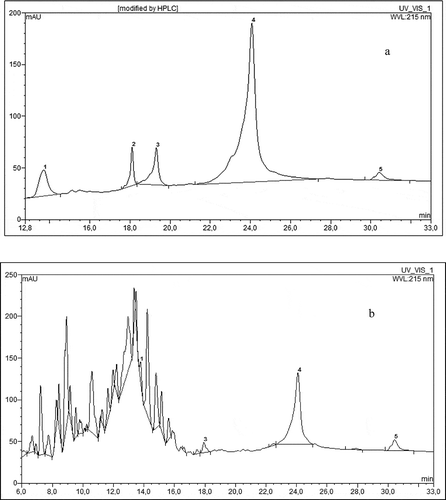
Figure 2 HPLC chromatogram of egg white protein amino acid profile (a) standard amino acids mixture, and (b) egg white protein.
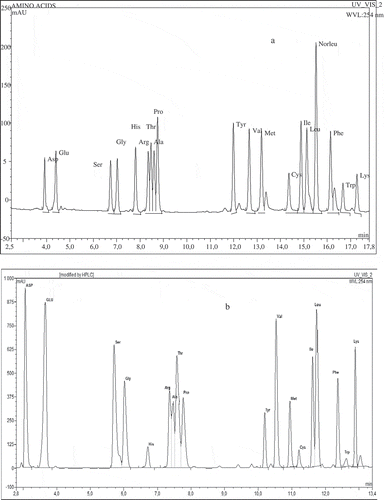
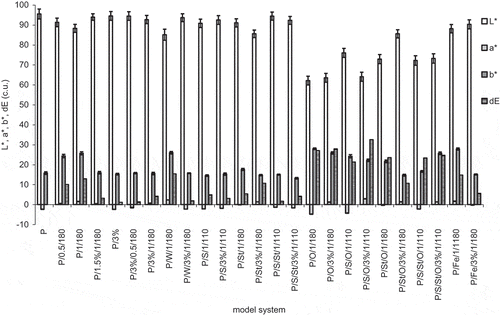
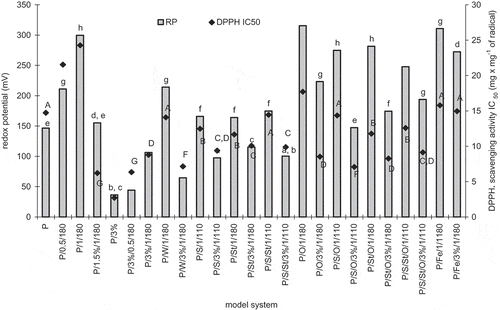
Figure 3 Total amino acids, protein content after heating, and pepsin in vitro digestibility of egg white protein in model systems with green coffee extract. Error bars show the relative standard deviation. Total amino acids are expressed on amount of protein added to the systems. Egg protein after heating means the amount of main protein fractions remaining after treatment determined by HPLC analysis. Egg white protein digestibility is a decrease of the amount of main protein fractions caused by 2 h pepsin digestibility determined by HPLC analysis.
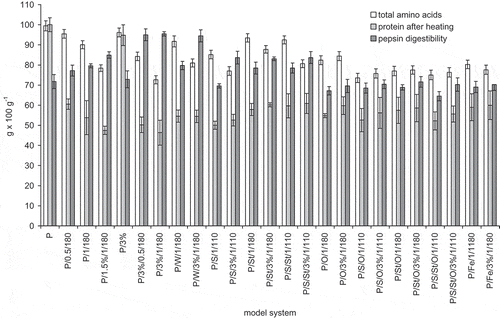
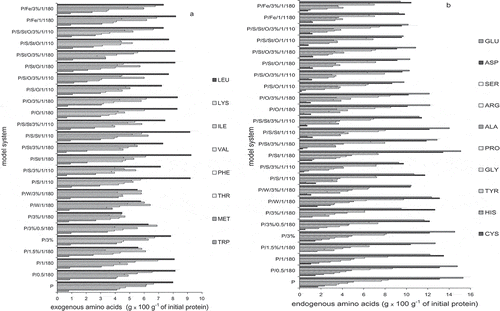
Figure 4 Amino acid profile of egg white protein in model systems with coffee extract (a) exogenous amino acids: leucine (LEU), lysine (LYS), isoleucine (ILE), valine (VAL), phenylalanine (PHE), threonine (THR), methionine (MET), and tryptophan (TRP), and (b) endogenous amino acids: glutamic acid + glutamine (GLU), aspartic acid + asparagine (ASP), serine (SER), arginine (ARG), alanine (ALA), proline (PRO), glycine (GLY), threonine (TYR), histidine (HIS), and cysteine + cystine (CYS). The relative standard deviation did not exceed 10%.