Figures & data
Table 1 Plackett-Burman design and its responses
Table 2 Variance analysis for Plackett-Burman design
Table 3 Box-Behnken design and its responses
Table 4 Test of significance for regression coefficient
FIGURE 1 Response curve for the immobilized lipase activity A: as an interaction between lipase added and adsorption time, B: as an interaction between lipase added and pH, and C: as an interaction between adsorption time and pH. Immobilized temperature 45°C, pH 4–8, adsorption time 3–5 h, lipase added at 20–40 mg/100 mg carrier, glutaraldehyde concentration 0.5%, and crosslinking time 2.5 h.
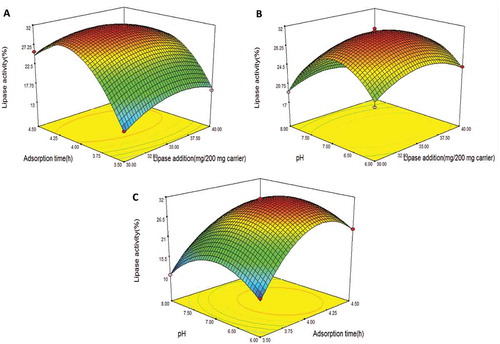
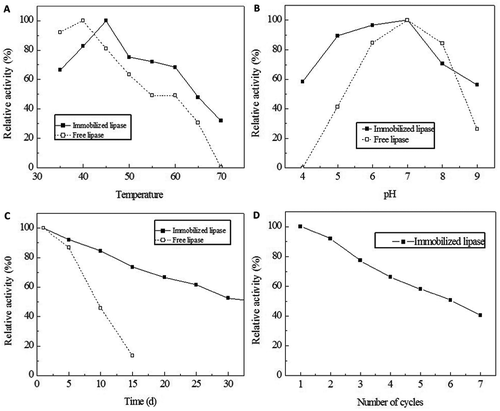
FIGURE 2 Physicochemical properties of the immobilized lipase. Thermal stability of free and immobilized Rhizopus oryzae lipase. The lipase was incubated for 1 h at a temperature range from 35 to 70°C in PBS (pH 6.8). The thermal stability was determined by measuring the lipase activity under each condition (A). The pH stability of the free and immobilized Rhizopus oryzae lipase. The lipase was incubated for 1 h at 45°C in PBS at the pH range from 4.0 to 9.0. The activity under each condition was measured (B). The storage stability of the free and immobilized Rhizopus oryzae lipase. The lipases were stored at 4°C in PBS (pH 6.8). The storage stability was tested by measuring the activity every 5 days until the activity was 50% of the initial activity (C). Reusability tests for the immobilized Rhizopus oryzae lipase. The reusability was evaluated by catalyzing several successive cycles. The residual activity was determined until the activity was 50% of the initial activity (D).