Figures & data
TABLE 1 Effect of Quercetin metabolites as major components of C. olitorius and V. vinifera on cyclooxygenase (COX-I) and cyclooxygenase (COX-II) inhibitory activity
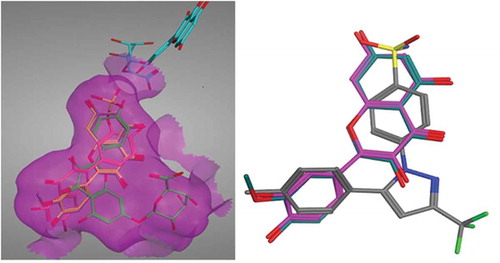
FIGURE 1 Overlay of the best scoring pose (left) for ibuprofen (A) and celecoxib (B) on their experimental crystal structures (right).
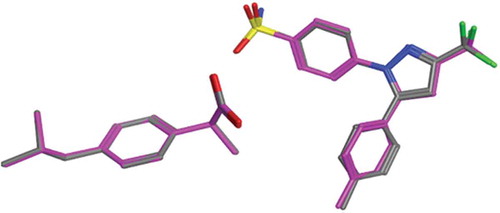
FIGURE 2 (Left): Superimposition of the active sites of both COX-I (cyano) and COX-II (magenta) showing the major structural differences resulting in a larger pocket in COX-II enzyme. (Right): COX-II active site consists of 3 main pockets: Pocket A formed of Phe367, Tyr371, Ser516, Trp373, and Leu370. Pocket B formed of Leu345, Arg106, Val102, Met99, Leu517, and Tyr341. Pocket C formed of Tyr341, Ser399, Phe504, Val509, and Leu338.
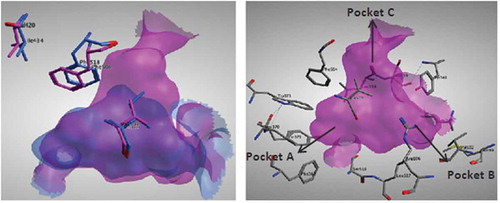
TABLE 2 Calculated docking scores for Quercetin metabolites into the active site of COX-I and COX-II enzymes
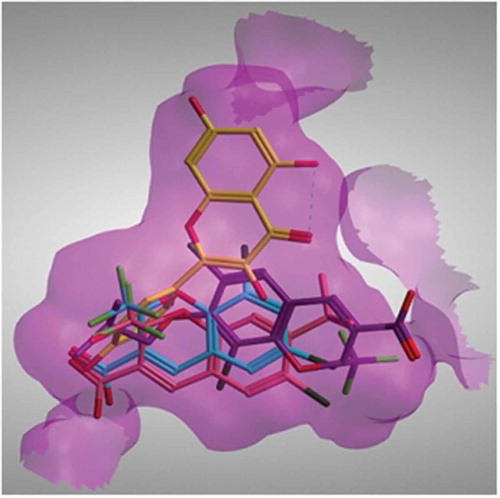
FIGURE 3 (Left): Quercetin and its metabolites docked inside COX-II active site. Quercetin (yellow), methyl quercetin (grey), quercetin sulphate (pink), and quercetin-7-glucuornide (green) all were able to bind to COX-II extra binding domain with the exception of quercetin-4-glucuornide (blue) which appears to be out of the pocket. (Right): Overlay of Celecoxib (grey), Quercetin (magenta), and Methyl quercetin (cyano) showing the benzopyran ring superimposed on the benzene sulphonamide moiety.