Figures & data
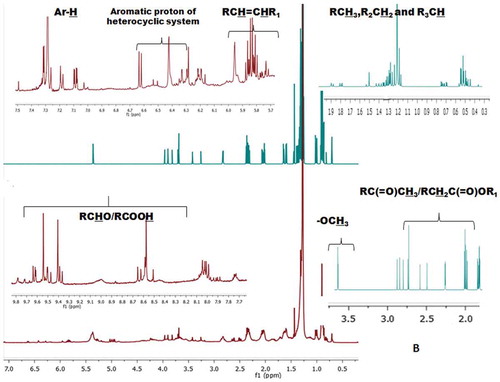
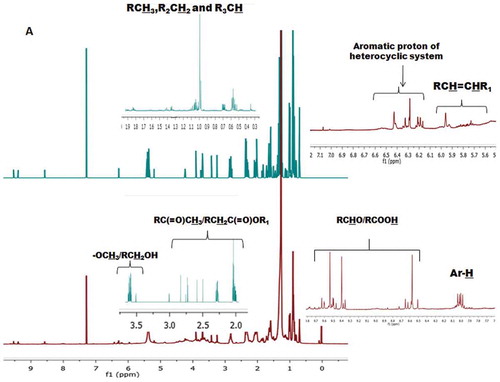
Table 1. Type and integral values of protons obtained from the 1H-NMR of the EM and CHCl3 fractions of seaweed S. wightii.
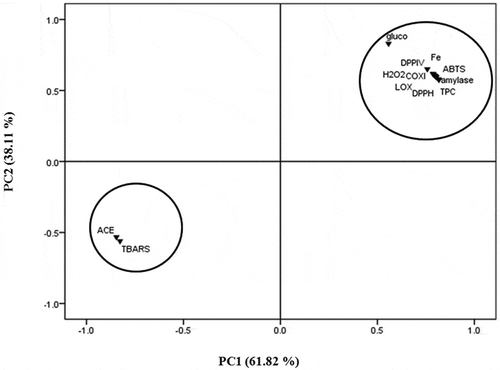
Table 2. Phenolic content, antioxidant, anti-inflammatory, antidiabetic, and ACE inhibitory activities of the EM and CHCl3 fractions of the seaweed S. wightii.
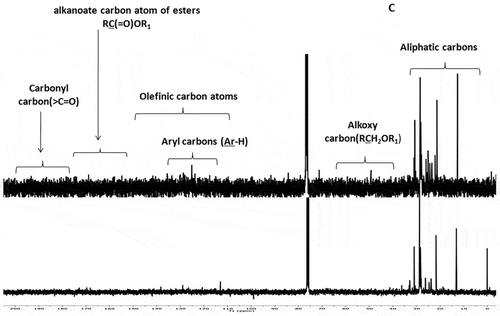
Figure 1. FT-IR spectra of A: EM and B: CHCl3 solvent fractions of S. wightii. The functional groups representing the distinct regions of the IR spectra were illustrated as (1) OHν of phenols and/or alcohols or N-Hν of amide groups, (2) >C=Oν of aldehydes or saturated aliphatic groups, (3) C-Cν of the aryl ring framework, and (4) C=Cν of olefinic groups. Stretching vibration has been indicated by “ν” as subscript to the functional group.
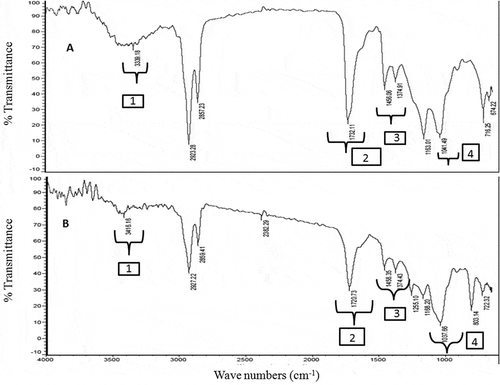
Figure 2b. CHCl3 solvent fractions of S. wightii. The protons at the defined regions of the 1H-NMR spectra were integrated to get the number of protons in specific regions.