Figures & data
Table 1. Mean and SD of effect of pH, temperature (°C), enzyme, NaCl, and CaCl2 concentration (mM) on rennet coagulation time (RCT) of fruits extract of Withania coagulans in 24 h of cultivation.
Table 2. Effect of milk temperature and milk pH on rennet coagulation time (RCT) of fruits extract of Withania coagulans in 24 h of cultivation.
Table 3. Mean ± SD of rennet coagulation time (RCT) and proteolytic activity of crude extract and AS fractions of fruits from Withania coagulanse.
Table 4. Effects of different protease inhibitors on rennet coagulation time (RCT) of enzymatic extract of Withania coagulans fruit as mean ± SD.
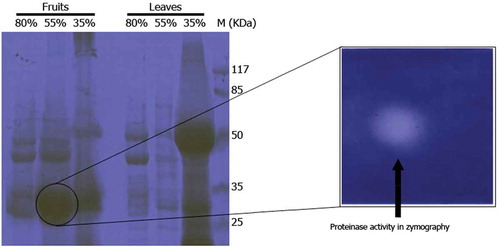
Figure 1. Comparison of casein hydrolysis activity by rennin, crude fruit’s, and leaves extracts of Withania coagulans (A) and rennin, crude leaves extract of W. coagulans and its ammonium sulfate (AS) fractions (B and C). Different types casein hydrolysis include αs-casein hydrolysis activity by rennin, crude fruit’s extract of W. coagulans and its AS fractions (D and E); β-casein hydrolysis activity by rennin, crude fruit’s extract of W. coagulans and its AS fractions (F) and κ-casein hydrolysis activity by rennin, crude fruit’s extract of W. coagulans and its AS fractions (G, H) are also show. SDS-PAGE (12%) was run and gel was stained with Coomassiee Briliant Blue R-250. C: casein; R: rennin; FE: fruit’s extract; LE: leaves extract.
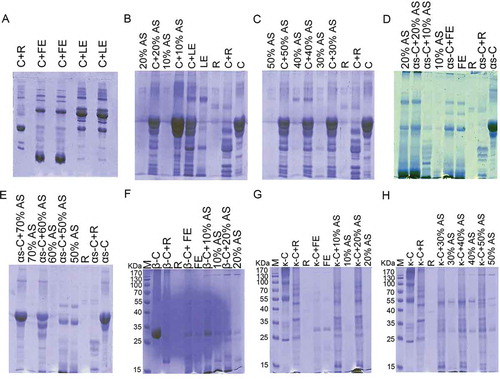
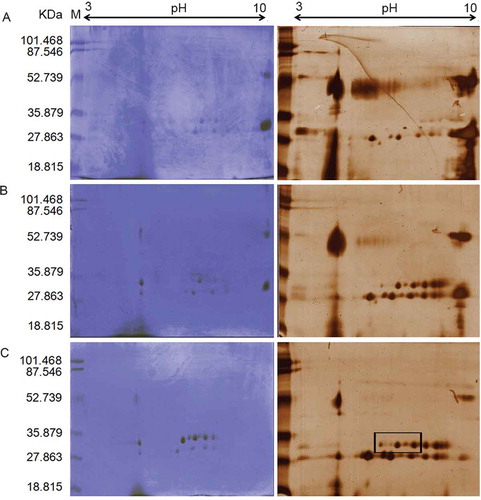
Figure 2. Two-dimensional electrophoresis pattern of A: 30%, B: 40%, and C: 50% ammonium sulfate (AS) fractions of fruit extracts of Withania coagulans. Proteins were separated by isoelectric focusing on IPG 3-10 NL as first dimension and then by 12% T SDS-PAGE in second dimension. Coomassiee Briliant BlueR-250 and silver nitrate staining were used for each %AS fraction.