Figures & data
Table 1. Salt substitutes (%) in surimi gels with, respectively, equivalent ionic strength (IS).
Table 2. Texture profile analysis (TPA) of surimi gels.
Figure 1. WHC of surimi gels with the addition of salt treatments. A1, A2, and A3; B1, B2, and B3; and C1, C2, and C3 were the surimi gels prepared with sodium, potassium, and calcium chloride corresponding to ionic strength of 0.51, 0.34, and 0.17, respectively. Bars on each column indicate standard deviation (SD). Different letters on the top of SD bars indicate significant differences (Duncan’s test, p < 0.05).
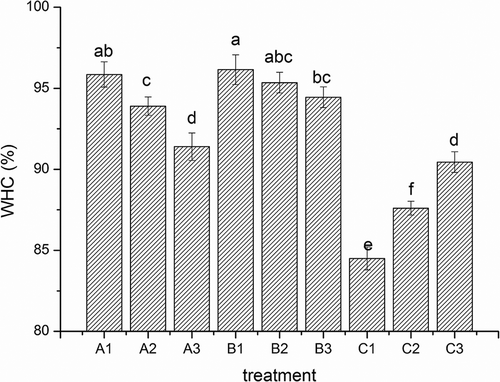
Figure 2. Breaking force, distance to rupture (a), and gel strength (b) of surimi gels with the addition of salt treatments. A1, A2, and A3; B1, B2, and B3; and C1, C2, and C3 were the surimi gels prepared with sodium, potassium, and calcium chloride corresponding to ionic strength of 0.51, 0.34, and 0.17, respectively. Bars on each column indicate standard deviation (SD). Different letters on the top of SD bars indicate significant differences (Duncan’s test, p < 0.05).
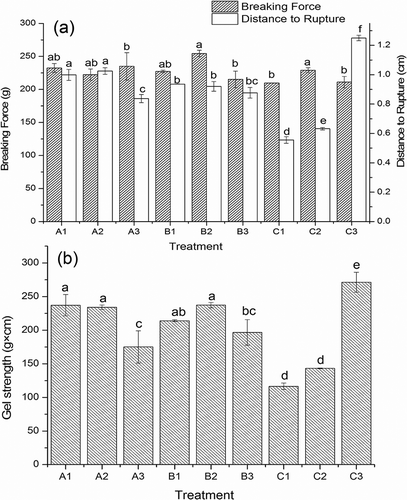
Figure 3. Rheological properties of surimi gel with the addition of salt treatments. A1, A2, and A3; B1, B2, and B3; and C1, C2, and C3 were the surimi gels prepared with sodium, potassium, and calcium chloride corresponding to ionic strength of 0.51, 0.34, and 0.17, respectively. (a) frequency sweep; (b) temperature sweep.
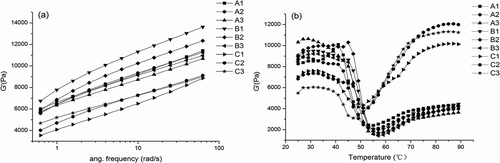
Figure 4. Chemical interactions of surimi gels with the addition of salt treatments. A1, A2, and A3; B1, B2, and B3; C1, C2, and C3 were the surimi gels prepared with sodium, potassium, and calcium chloride corresponding to ionic strength of 0.51, 0.34, and 0.17, respectively. Bars on each column indicate standard deviation (SD). Different letters on the top of SD bars indicate significant differences (Duncan’s test, p < 0.05). (a) nonspecific associations; (b) ionic bonds; (c) hydrogen bonds; and (d) hydrophobic interactions.
Figure 5. Sulfhydryl groups’ contents of surimi gels with the addition of salt treatments. A1, A2, and A3; B1, an d B2, B3; and C1, C2, and C3 were the surimi gels prepared with sodium, potassium, and calcium chloride corresponding to ionic strength of 0.51, 0.34, and 0.17, respectively. Bars on each column indicate standard deviation (SD). Different letters on the top of SD bars indicate significant differences (Duncan’s test, p < 0.05).
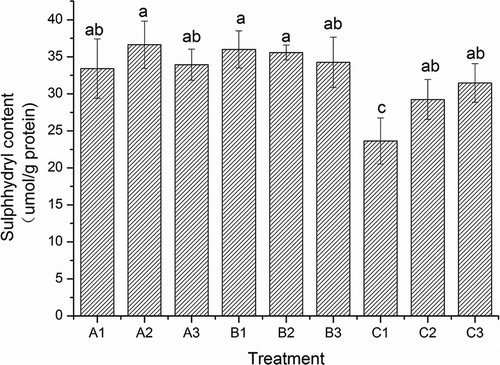
Figure 6. Solubility of surimi gels with the addition of salt treatments. A1, A2, and A3; B1, B2, and B3; an d C1, C2, and C3 were the surimi gels prepared with sodium, potassium, and calcium chloride corresponding to ionic strength of 0.51, 0.34, and 0.17, respectively. Bars on each column indicate standard deviation (SD). Different letters on the top of SD bars indicate significant differences (Duncan’s test, p < 0.05).
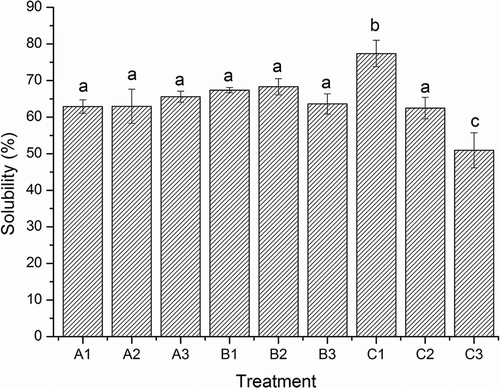
Figure 7. Scanning electronic microscopy (SEM) of surimi gels with the addition of salt treatments. Bar represents 10 μm. A1, A2, and A3; C1, C2, and C3 were the surimi gels prepared with sodium and calcium chloride corresponding to ionic strength of 0.51, 0.34, and 0.17, respectively (magnification, 10,000×). C3a and C3b were graphs of C3 with magnification for 20,000× and 40,000×, respectively.
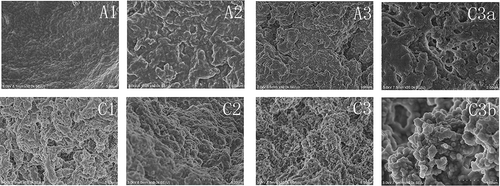
Table 3. Correlation coefficient between gel properties and chemical interactions of surimi gels.
