Figures & data
Table 1. L*, a*, and b* value of non-oxidized and oxidized Sheldrake breast muscle.
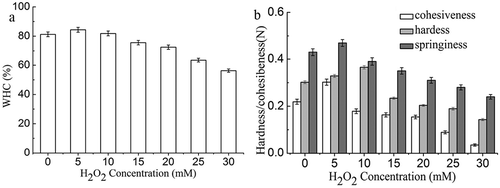
Figure 1. WHC (a), shear force (b), and TBARS value (c) of non-oxidized and oxidized Sheldrake breast muscle.
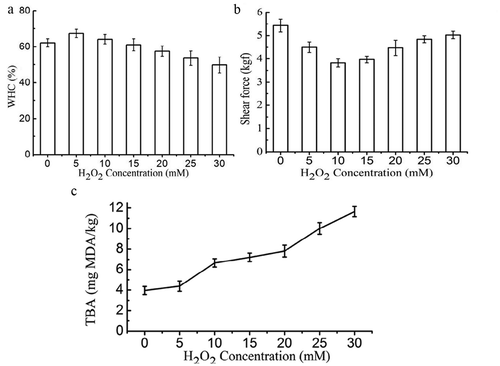
Figure 2. Protein carbonyls (a), sulfhydryl group contents (b), and the surface hydrophobicity (c) of non-oxidized and oxidized MP.
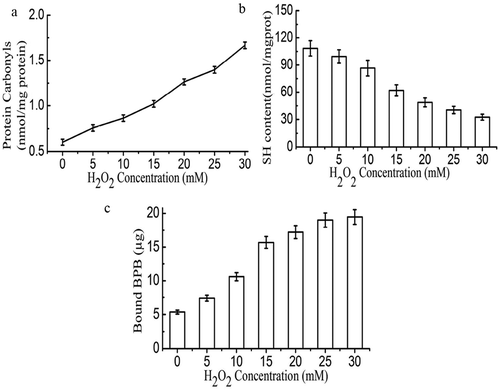
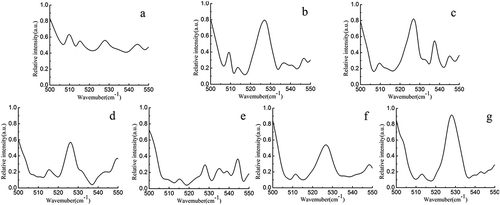
Figure 4. Raman spectra (500–550 cm–1 region) of non-oxidized (a) and oxidized MP gels treated with different HRGS (5 mM H2O2 (b); 10 mM H2O2 (c); 15 mM H2O2 (d); 20 mM H2O2 (e); 25 mM H2O2 (f); 30 mM H2O2 (g)).