Figures & data
Table 1. Retention times, UV–Vis, mass spectral data, and contents of phenolic compounds in bilberry and bog bilberry.
Table 2. Retention times, UV–Vis, mass spectral data, and contents of phenolic compounds (mg/100 g DW) in bilberry and bog bilberry leaf extracts.
Figure 1. HPLC/DAD chromatograms of berry extracts of (1) bilberry (in red) and (2) bog bilberry (in green): (a) 520 nm, (b) 360 nm, (c) 300 nm.
A1–A17 for anthocyanins, Ac1–Ac14 for hydroxycinnamic acid derivatives, F1–F15 for flavonols, S1–S2 for stilbenes, and I1 for iridoid. Peak assignments as in .
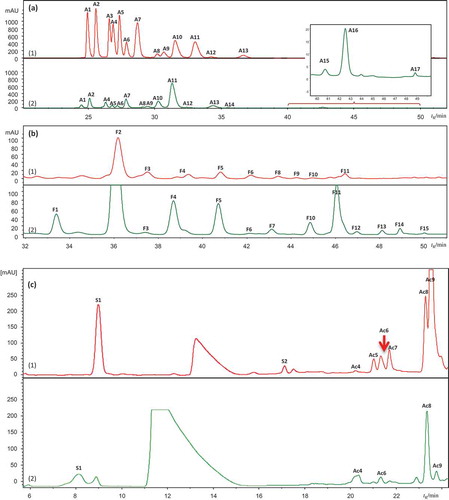
Figure 2. HPLC/DAD chromatograms of leaf extract of (1) bilberry (in red) and (2) bog bilberry (in green): (a) 280 nm, (b) 360 nm.
Ac1–Ac13 for hydroxycinnamic acid derivatives, F1–F15 for flavonols, FL1–FL5 for flavan-3-ols, S1–S2 for stilbenes, I1–I2 for iridoids, and C for cinchonain. Peak assignments as in
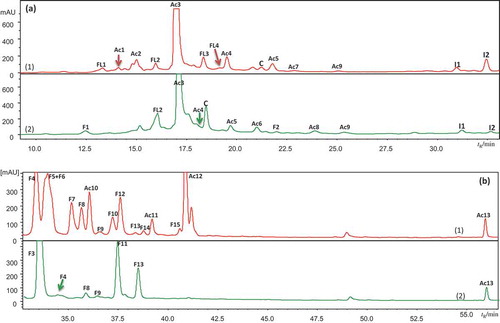
Figure 3. Distribution of the main phenolic classes in (a) berries and (b) leaves of bilberry and bog bilberry.
Ac: acid derivatives; I: iridoids; S: stilbenes; F: flavonols; FL: flavan-3-ols; A: anthocyanins; Ci: cinchonain; D: delphinidin derivatives; C: cyanidin derivatives; Pt: petunidin derivatives; Pe: peonidin derivatives; M: malvidin derivatives; Q: quercetin derivatives; My: myricetin derivatives; L: laricitrin derivatives; Sy: syringetin derivatives; K: kaempferol derivatives; Is: isorhamnetin derivatives; Vm: Vaccinium myrtillus – bilberry; Vu: Vaccinium uliginosum – bog bilberryx axis – contents of phenolic compounds, anthocyanins, and flavonols expressed as mg/100 g FW in berries and as mg/100 g DW in leaves
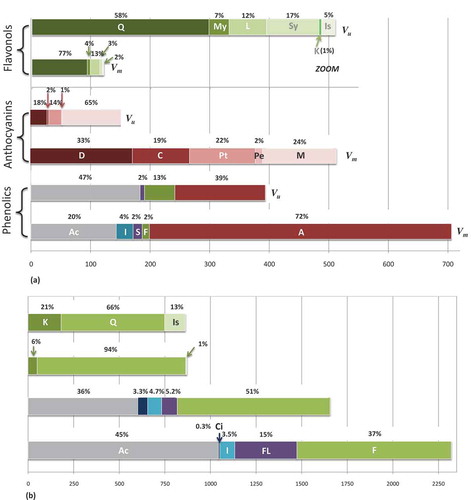
Table 3. Content of macro- and microelements in berries and leaves of bilberry and bog bilberry.
